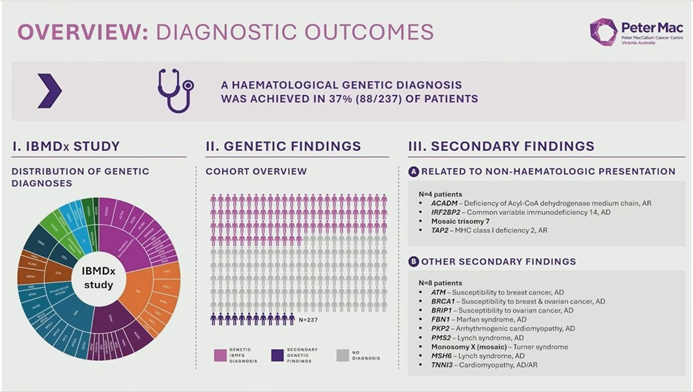
Breaking Diagnostic Bottlenecks: The IBMDX Study Reveals 37% Diagnostic Yield and Health Economic Value of Whole Genome Sequencing (WGS) in Inherited Bone Marrow Failure Syndromes
At the 2025 American Society of Hematology (ASH) Annual Meeting, Dr. Lucy Fox from the Peter MacCallum Cancer Centre (Australia) presented the highly anticipated results of the IBMDX study. This landmark trial evaluated the clinical utility and economic impact of "Upfront Whole Genome Sequencing (WGS)" in patients with suspected Inherited Bone Marrow Failure Syndromes (IBMFS). The findings suggest a paradigm shift is underway: WGS not only achieves a diagnostic yield of 37%—surpassing traditional panels and exome sequencing—but also uncovers complex genomic mechanisms previously invisible to standard testing.