Editor’s Note: During the 2023 European Society for Medical Oncology (ESMO) Annual Meeting, a Phase II multi-center clinical trial’s results, led by Academician Xu Binghe and his team from the Cancer Hospital of the Chinese Academy of Medical Sciences, will be presented in a poster session (Abstract #404P). The trial demonstrates the excellent efficacy of the domestically developed innovative CDK4/6 inhibitor Birociclib as a monotherapy in the treatment of HR+/HER2- metastatic breast cancer after multiple lines of treatment, including endocrine therapy and chemotherapy. Dr. Jiayu Wang from the Cancer Hospital of the Chinese Academy of Medical Sciences provides an in-depth analysis of these research findings.
Birociclib is a selective inhibitor of cyclin-dependent kinases (CDK) 4 and 6, and this Phase II study (NCT04539496) aimed to evaluate the efficacy and safety of Birociclib as a monotherapy for refractory HR+/HER2- metastatic breast cancer (MBC). The data reported at ESMO is the result of Independent Review Committee (IRC) assessment, following the previously reported investigator assessment data at ASCO.
Key Findings:
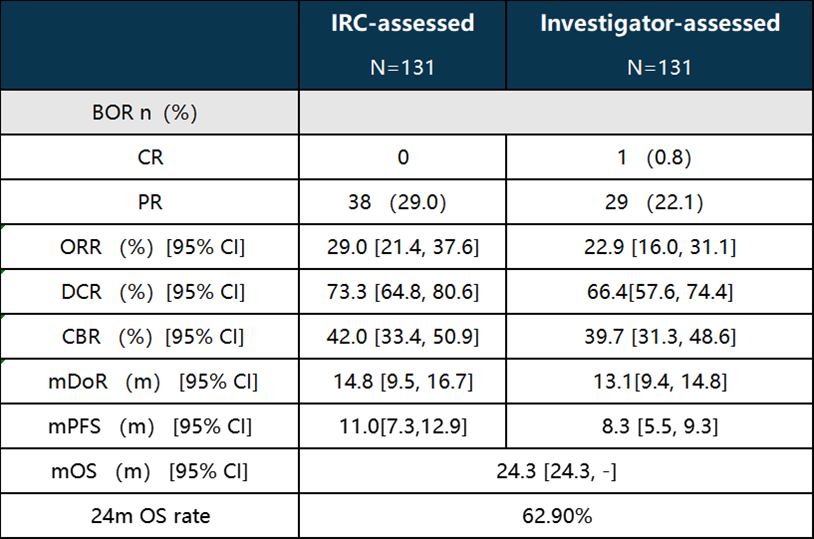
1. Birociclib monotherapy for HR+/HER2- metastatic breast cancer patients demonstrated significant efficacy. The IRC-confirmed objective response rate (ORR) was 29%, with a median duration of response (DoR) of 14.8 months, a median progression-free survival (PFS) of 11 months, and an overall survival (OS) of 24.3 months, with a 24-month OS rate of 62.9%.
2. Birociclib was beneficial for HR+/HER2- breast cancer patients with visceral and liver metastases, with IRC-assessed ORRs of 26.1% and 21.2%, respectively. Subgroup analysis results were consistent with the overall findings.
3. The most common treatment-emergent adverse events (TEAE) during therapy were related to the gastrointestinal and hematologic systems, mostly grade 1-2, and most of them were manageable and resolved. Birociclib demonstrated good tolerability.
Researchers’ Comments:
Visceral metastases and resistance to endocrine therapy are significant challenges in the treatment of HR+/HER2- advanced breast cancer. A series of studies, such as PALOMA, MONARCH, and MONALEESA, have demonstrated that CDK4/6 inhibitors can delay or reverse endocrine resistance, and patients with visceral metastases can also benefit from CDK4/6 inhibitor treatment, significantly changing the treatment landscape for HR+/HER2- advanced breast cancer.
Birociclib, an innovative CDK4/6 inhibitor developed by the Chinese pharmaceutical company Xuanzhu Biotech, is structurally similar to abemaciclib and belongs to the benzimidazole series of molecules. In vitro enzymatic data show that Birociclib can effectively inhibit the kinase activity of CDK4 and CDK6 and exhibits good selectivity for these kinases.
In this Phase II clinical study presented at ESMO, patients included those with HR+/HER2- breast cancer who had progressed after multiple lines of treatment, including endocrine therapy and chemotherapy, and many had visceral metastases. The IRC assessment showed an ORR of 29% and a median PFS of 11 months. This is an improvement over previously reported investigator assessment results (median PFS: 8.3 months, ORR: 22.9%). In a similar study of the drug abemaciclib as a monotherapy (Phase II MONARCH 1 trial), baseline characteristics of the patient population were similar to this study, with an IRC-assessed ORR of 17.4% and a median PFS of 5.9 months. In terms of safety, the most common TEAEs for Birociclib at a dose of 480mg twice daily were diarrhea (93.1%), neutropenia (87.0%), and leukopenia (86.3%), with most TEAEs resolving or improving after dose interruption or reduction. Overall, the study data highlight the advantages of Birociclib monotherapy in advanced breast cancer patients who have progressed after multiple lines of treatment, providing new insights and options for the treatment of advanced HR+/HER2- breast cancer patients.
Based on the results of this study, the application for the approval of Birociclib monotherapy for advanced breast cancer has been accepted by the National Medical Products Administration (NMPA). Additionally, the results of a Phase III clinical trial of Birociclib in combination with fulvestrant (NCT05077449) have been submitted and accepted. Furthermore, a Phase III clinical trial (NCT05257395) of Birociclib in combination with aromatase inhibitors for the treatment of HR+/HER2- advanced metastatic breast cancer is currently underway. With the release of more data, it is believed that Birociclib will bring further benefits to HR+/HER2- breast cancer patients in China.
References:
A multicenter, single-arm, open-label trial of Birociclib, a CDK4/6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. 2023 ESMO. Abstract #404P.
Maura N. Dickler, et al. MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin Cancer Res. 2017 September 01; 23(17): 5218–5224. doi: 10.1158/1078-0432.CCR-17-0754.

Dr. Jiayu Wang
Department of Internal Medicine, Cancer Hospital of the Chinese Academy of Medical Sciences

