In January 2023, a study led by Professor Meng Zhao from Zhongshan School of Medicine was published in the prestigious international academic journal ——Nature Cell Biology (IF=21.3). The title of the study is "PD-1 signalling defines and protects leukaemic stem cells from T cell receptor-induced cell death in T cell acute lymphoblastic leukaemia." This research presents significant findings on the role of PD-1 signaling in defining and protecting Leukaemic Stem Cells (LSCs) in T cell acute lymphoblastic leukaemia (T-ALL). The study not only elucidates the complex dynamics of disease initiation and progression but also holds the potential for developing novel therapeutic interventions. This research was also recently recognized as one of the "Top Ten Advances in Hematology in China in 2023."
T-cell acute lymphoblastic leukemia (T-ALL) emerges as a formidable adversary within the spectrum of blood cancers, marked by the rapid proliferation of immature T-cell progenitors. The imperative to unravel the mysteries encompassing leukaemic stem cells (LSCs) and formulate effective strategies for their targeted elimination remains pivotal for advancing prognosis and elevating treatment outcomes. Zhou et al. present a groundbreaking revelation regarding the profound role of PD-1 signaling in both defining and safeguarding LSCs in T-ALL. This research not only illuminates the intricate dynamics of disease initiation and progression but also holds tremendous promise for the development of novel therapeutic interventions.
In their endeavor to unravel the hierarchy of T-ALL, Zhou et al. adopt a sophisticated and multifaceted approach. The integration of limiting dilution assays, and in vivo live imaging enables the identification of a discrete population of cells expressing the inhibitory receptor programmed cell death 1 (PD-1) at the apex of the leukemia hierarchy. Strikingly, PD-1+ cells emerge as functionally significant LSCs, challenging prevailing notions and offering a groundbreaking perspective on the disease’s cellular hierarchy.
The study delves further into the functional significance of PD-1 signaling in maintaining and protecting LSCs against T cell receptor (TCR)-induced apoptosis. PD-1+ LSCs exhibit heightened NOTCH1-MYC activity, a crucial contributor to disease initiation, therapy resistance, and relapse in T-ALL. The intricate interplay between PD-1 signaling and the NOTCH1-MYC pathway emerges as a key determinant shaping the fate of T-ALL. Importantly, the researchers demonstrate that disrupting PD-1 signaling, either through genetic deletion or antibody therapy, leads to a significant eradication of LSCs and a suppression of disease progression in both mouse models and patient-derived xenografts.
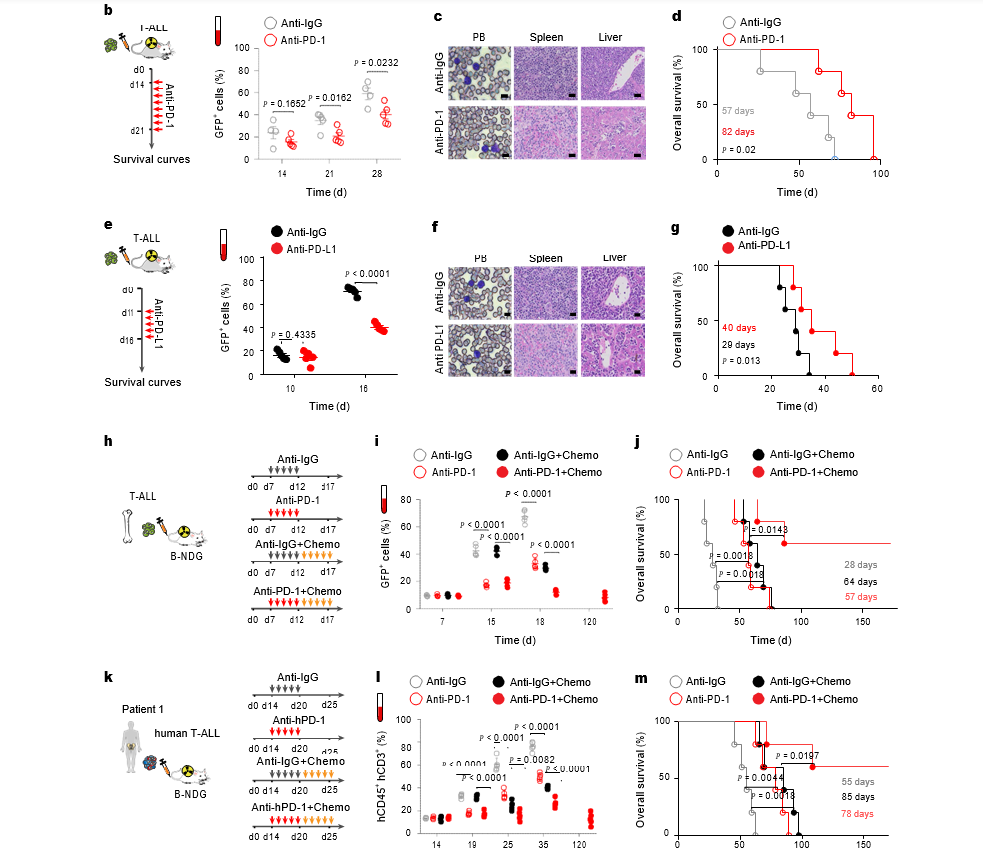
Translating these laboratory findings into a clinical context, the study reveals that high levels of PD-1 expression in T-ALL patients correlate with poor disease prognosis and reduced survival. This observation not only underscores the clinical relevance of PD-1 as a prognostic marker but also suggests that assessing PD-1 levels could guide treatment decisions. The study further illuminates the potential of combination therapy involving PD-1 blockade and chemotherapy, demonstrating extended survival in murine models engrafted with T-ALL cells.
The study’s emphasis on transparency in reporting statistical analyses beckons researchers to provide exhaustive details about covariates tested, adjustments made for assumptions or restrictions, and thorough descriptions of estimates used in regression analyses. The meticulous reporting of negative results, including sample sizes and the number of biological repeats, contributes to a more comprehensive understanding of the research landscape.
In conclusion, the study has unveiled a paradigm-shifting understanding of T-ALL, spotlighting the crucial role of PD-1 signaling in defining and protecting LSCs. This newfound knowledge not only provides a potential prognostic marker for clinical decision-making but also opens up exciting avenues for the development of targeted therapies. Transparency in reporting statistical analyses and detailed methodologies ensures the reliability and reproducibility of research findings, fostering a more robust foundation for future studies.
The intricate interplay between PD-1 signaling and the NOTCH1-MYC pathway adds layers to our understanding of T-ALL biology, beckoning researchers to further explore these molecular intricacies. As we navigate the future of T-ALL research, this study hold the promise of improving outcomes for patients grappling with this aggressive form of leukemia.


