Editor's Note: Triple-negative breast cancer (TNBC) refers to a subtype of breast cancer where the tumor cells lack expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounting for approximately 15-20% of all breast cancer cases. This subtype shows poor response to endocrine therapy and HER2-targeted therapy. In recent years, there have been significant advancements in the treatment of TNBC patients with immunotherapy and Trop-2 targeted therapy, bringing about many breakthroughs in the field of TNBC treatment. In this article, Professor Jiang Zefei from the Department of Oncology at the People's Liberation Army General Hospital provides insights into the recent developments in the treatment of triple-negative breast cancer patients.Tumor Outlook: There have been several notable research findings in the field of TNBC treatment in 2023. Could you please review and interpret them for us?
Professor Jiang Zefei: Over the past year, there have been significant advancements in the treatment of both early-stage and advanced TNBC patients. One of the most important advancements in the treatment of early-stage TNBC patients is the updated follow-up data from the KEYNOTE-522 study. The KEYNOTE-522 study is a phase III clinical trial designed to evaluate the efficacy and safety of pembrolizumab plus neoadjuvant chemotherapy (pembrolizumab group) compared to neoadjuvant chemotherapy alone, followed by pembrolizumab or placebo adjuvant therapy, in early-stage TNBC patients. The study has dual primary endpoints, including pathological complete response (pCR; ypT0/Tis ypN0) and event-free survival (EFS; time from randomization to disease progression [inoperable definitive surgery], local/distant recurrence, second primary cancer, or death from any cause).
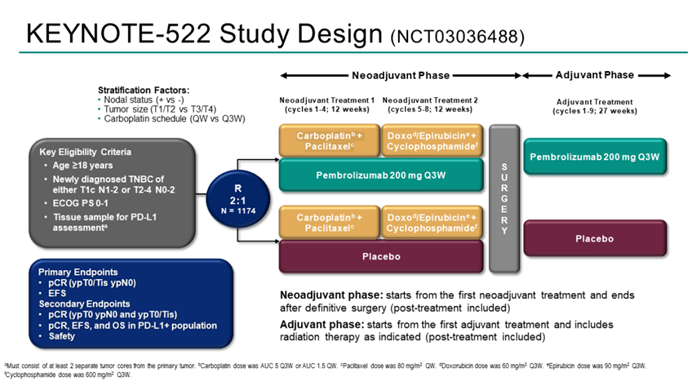
The previous data from the KEYNOTE-522 study demonstrated that the addition of pembrolizumab to neoadjuvant chemotherapy significantly increased the pathological complete response (pCR) rate in early-stage TNBC patients. In March 2022, data with a median follow-up of 39.1 months showed a statistically and clinically significant improvement in event-free survival (EFS) in the pembrolizumab group. At the 2023 ESMO Congress, data with a median follow-up of 63.1 months from the KEYNOTE-522 study were presented, revealing that 145 patients (18.5%) in the pembrolizumab group experienced an EFS event, compared to 108 patients (27.7%) in the placebo group (HR 0.63; 95% CI: 0.49-0.81). Pembrolizumab continued to demonstrate clinically significant improvement in EFS for high-risk early-stage TNBC patients compared to placebo. Consistent EFS benefits were observed regardless of PD-L1 expression and lymph node involvement. In pre-specified, non-randomized exploratory analysis, the 5-year EFS rates were 92.2% and 88.2% (for patients achieving pCR) and 62.6% and 52.3% (for patients not achieving pCR) in the pembrolizumab group and placebo group, respectively. Overall survival (OS) follow-up is still ongoing.
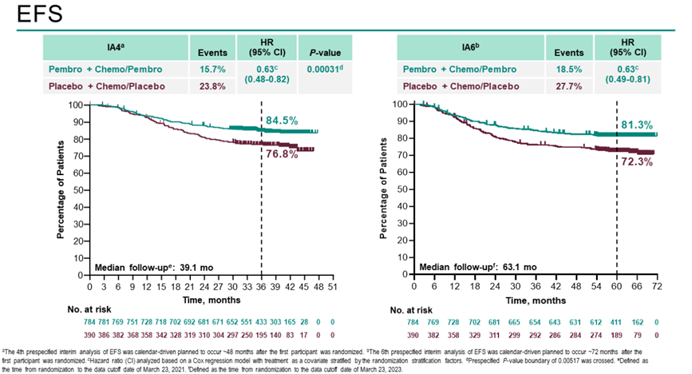
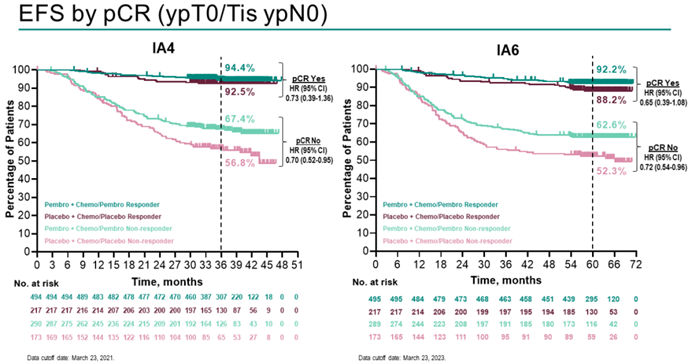
The KEYNOTE-522 study has opened the door to neoadjuvant immunotherapy for TNBC. It is the first phase III randomized controlled trial evaluating the combination of pembrolizumab with chemotherapy for neoadjuvant/adjuvant treatment of early-stage TNBC. The results of the study have shown that immunotherapy combined with chemotherapy can greatly improve patient survival. Moreover, an increase in pCR rates has been observed across different PD-L1 expression strata, making pembrolizumab the earliest approved immunotherapy drug for early-stage high-risk TNBC patients.
IMpassion 031 is a global, multicenter, randomized, double-blind phase III clinical trial designed to evaluate the efficacy and safety of atezolizumab in combination with chemotherapy for neoadjuvant treatment of early-stage TNBC. The final analysis data of the IMpassion 031 study were presented at the 2023 ESMO Breast Cancer Congress. The results demonstrated that regardless of patients’ PD-L1 expression status, the atezolizumab combined with chemotherapy regimen could increase pCR rates, EFS, DFS, and overall survival (OS), improving patients’ survival prognosis, with good safety profiles.
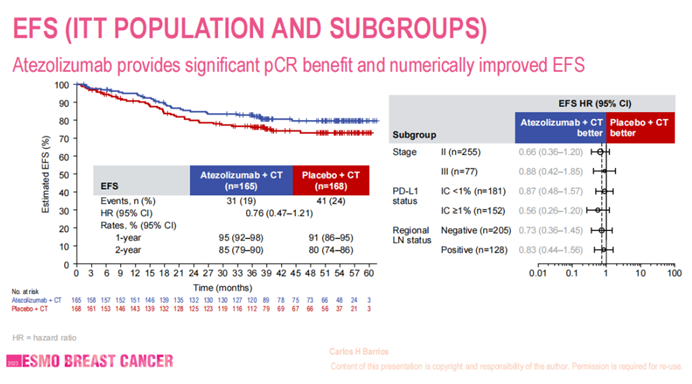
Currently, there is a consensus among international and domestic experts and guidelines recommending the combination of immunotherapy with chemotherapy for eligible early-stage TNBC patients. With the improvement in treatment efficacy, chemotherapy plus immunotherapy has become the standard regimen for neoadjuvant treatment of TNBC patients.
Tumor Outlook: Apart from the advancements in the treatment of early-stage TNBC patients, there have been significant developments in the field of advanced TNBC over the past year, and the TORCHLIGHT study led by you has also achieved promising results. Could you please introduce it to us?
Professor Jiang Zefei: With the collective efforts and perseverance of all team members and patients involved in the TORCHLIGHT study, the data from the TORCHLIGHT study were presented at the 2023 ASCO Annual Meeting as a Late-Breaking Abstract (LBA), for which we are immensely grateful. Recently (January 8, 2024), the results of the TORCHLIGHT study were published in the top-tier international journal, Nature Medicine (IF=83), once again gaining international academic recognition and allowing more patients to see the benefits of toripalimab combined with nab-paclitaxel. Even today, we continue to see some patients benefiting from this treatment. The study integrates clinical issues, sound research methodology, excellent products, and the wisdom of Chinese scholars. We believe this regimen will soon be approved for indications and incorporated into guidelines, benefiting more patients.
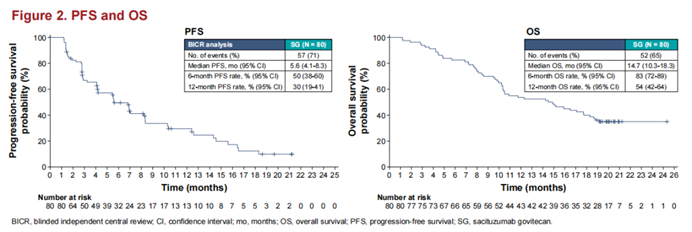
In addition to the TORCHLIGHT study, in the field of advanced TNBC treatment, the follow-up results of the KEYNOTE-355 study have also demonstrated the sustained benefits of chemotherapy combined with immunotherapy for patients. The emergence of novel ADC drugs has also brought more treatment hope to patients with advanced TNBC. In TNBC patients with low HER2 expression, DS-8201 (T-DXd) has extended PFS for patients. The EVER-132-001 study, led by Academician Xu Binghe’s team, with participation from 15 domestic centers and scholars, updated results presented at the 2023 SABCS Congress. With a median follow-up of 14.7 months (range 1.2-25.3), Chinese patients treated with SG (trastuzumab deruxtecan) had a median PFS of 5.6 months and a median OS of 14.7 months, consistent with the survival benefits reported in the ASCENT study, with overall safety results also similar.
SKB264 is also an antibody-drug conjugate, consisting of an anti-Trop-2 antibody coupled with a cytotoxic derivative of belotecan via a novel linker. The latest efficacy and safety data from the SKB264 treatment for metastatic TNBC (mTNBC), led by Professor Yin Yongmei’s team at Jiangsu Provincial People’s Hospital, have demonstrated sustained relief and long-term OS benefits for TNBC patients. The latest efficacy and safety data presented at the 2023 SABCS Congress showed that 59 treated mTNBC patients received SKB264 monotherapy administered once every two weeks. With a median follow-up of 22.8 months (95% CI: 21.3-25.2), the ORR was 42.4% (25/59, with 22 cases confirmed and 3 cases unconfirmed), and the disease control rate (DCR) was 76.3% (45/59). The median duration of response (DoR) was 11.5 months (3.7-22.1+). The median progression-free survival (mPFS) was 5.7 months (95% CI: 3.8-9.1). The median overall survival (mOS) was 16.8 months (95% CI: 12.7-NE), with 12-month and 24-month overall survival rates of 65.0% and 39.5%, respectively. Updated data indicate that mTNBC patients who have undergone multiple-line treatments can benefit from sustained relief and long-term OS with SKB264 treatment, with manageable safety, and patients with high Trop-2 expression demonstrate a higher response rate.
In the field of advanced TNBC patient treatment, the related studies of immunotherapy combined with chemotherapy, ADC drugs, and others, published in 2023, have all shown promising treatment effects, providing more options for TNBC patient treatment. It is believed that with the further development of international and domestic clinical studies on advanced TNBC, we can explore better opportunities for immunotherapy and more effective combination immunotherapy drugs in the future. Additionally, we will conduct corresponding translational research to identify relevant biomarkers to guide clinical diagnosis and treatment for patients and improve patient clinical management.


