In April 2023, a study led by Professor Jun Zhu from Peking University Cancer Hospital and Institute was published in the prestigious international academic journal ——Blood (IF=25.669). The title of the study is "Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial". This study findings demonstrate consistent efficacy and safety of Pola-R-CHP versus
R-CHOP in the Asian and global populations in POLARIX.
In an enlightening development within the realm of oncology, a recent study titled “Polatuzumab Vedotin in Previously Untreated DLBCL: An Asia Subpopulation Analysis from the Phase 3 POLARIX Trial” has unveiled significant findings that promise to refine the treatment landscape for patients suffering from diffuse large B-cell lymphoma (DLBCL). Conducted across various Asian countries, this pivotal research delves into the comparative efficacy and safety of polatuzumab vedotin combined with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) against the standard rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) regimen in newly diagnosed DLBCL patients.
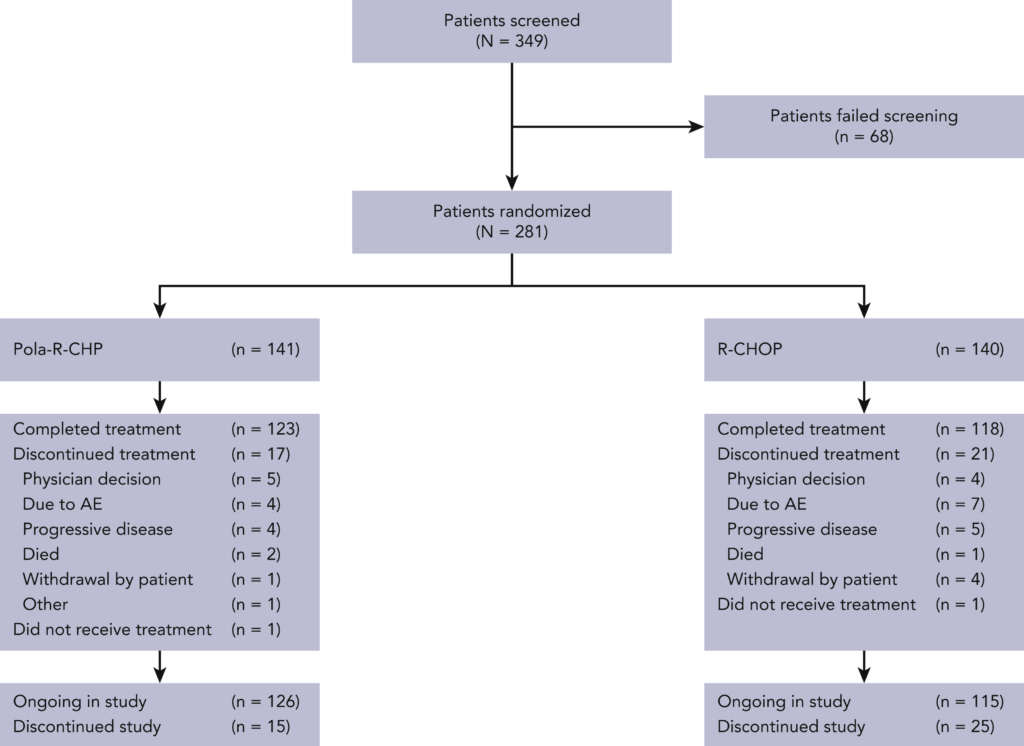
The core objective of the study was to assess whether the progression-free survival (PFS) benefits observed with Pola-R-CHP in the global population could be replicated within the Asian subpopulation, thereby providing a robust treatment alternative tailored to diverse genetic and environmental backgrounds. A total of 281 patients from Asia, comprising 160 from the intent-to-treat (ITT) global study population and an additional 121 from a China extension cohort, were enrolled to participate in this rigorous investigation.
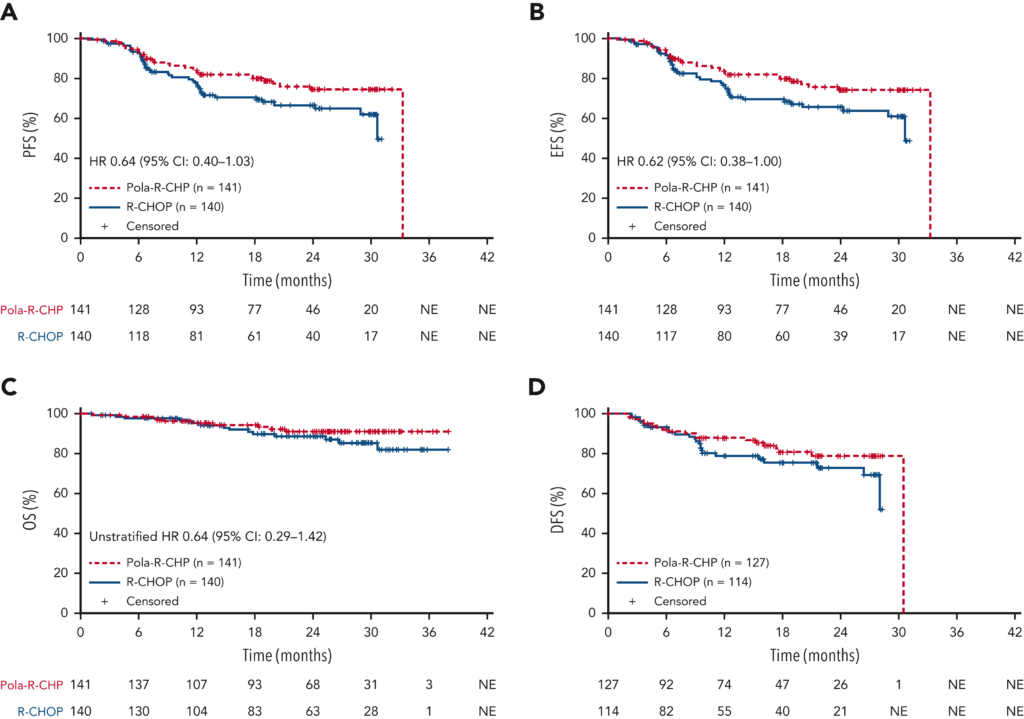
The primary efficacy endpoint was progression-free survival (PFS) as assessed by investigators. The study’s results were highly promising, revealing that Pola-R-CHP significantly improved PFS over R-CHOP, with a hazard ratio (HR) of 0.64 favoring the Pola-R-CHP regimen. Notably, the two-year PFS rate was 74.2% for patients treated with Pola-R-CHP, compared to 66.5% for those receiving R-CHOP.
Secondary endpoints, including event-free survival (EFS), the complete response rate at the end of treatment (EOT), overall survival (OS), and disease-free survival (DFS) in patients who achieved a complete response (CR), echoed the primary endpoint’s findings. These results underscored the enhanced efficacy of Pola-R-CHP across multiple critical measures of treatment success.
The study also meticulously evaluated the safety profile of Pola-R-CHP in comparison to R-CHOP. The findings indicated that Pola-R-CHP’s safety profile was comparable to that of R-CHOP, with similar rates of grade 3-4 adverse events (AEs) and serious AEs. Among the most commonly reported adverse events were anemia, alopecia, and a decrease in neutrophil count. Remarkably, the incidence of peripheral neuropathy (PN), a significant concern in cancer treatment, was lower in patients treated with Pola-R-CHP than those in the global study population, suggesting a potential advantage in terms of tolerability.
The “Polatuzumab Vedotin in Previously Untreated DLBCL: An Asia Subpopulation Analysis from the Phase 3 POLARIX Trial” study presents compelling evidence that supports the use of Pola-R-CHP as a highly effective and safe treatment option for previously untreated DLBCL patients, including those in Asia. The consistent efficacy and manageable safety profile of Pola-R-CHP, as demonstrated in this subpopulation analysis, affirm its potential as a valuable addition to the therapeutic arsenal against DLBCL. This study not only underscores the importance of tailored treatment approaches based on regional patient populations but also marks a significant step forward in the quest for optimizing DLBCL patient outcomes globally.


