At the recently concluded American Society of Hematology (ASH) Annual Meeting in 2025, Dr. Hanny Al-Samkari from Massachusetts General Hospital presented the highly anticipated primary analysis of the VAYHIT 2 study. Presented as a Late-Breaking Abstract (LBA), this global, randomized, double-blind, Phase 3 clinical trial evaluated the efficacy and safety of combining the novel BAFF receptor antagonist Ianalumab with Eltrombopag in patients with primary immune thrombocytopenia (ITP). The results signal a potential paradigm shift for patients who have failed first-line corticosteroid therapy, offering a new avenue for durable disease control.01. The “Untapped Gold Mine”: Targeting the BAFF Pathway
Primary ITP is characterized by autoimmune platelet destruction. While first-line corticosteroids are standard, most adults relapse and face a chronic disease course requiring indefinite maintenance therapies like thrombopoietin receptor agonists (TPO-RA). As the disease progresses, the autoimmune phenotype complexifies, making treatment increasingly difficult.
Dr. Al-Samkari highlighted the B-cell activating factor (BAFF) signaling pathway as a critical, yet previously unexploited, therapeutic target. BAFF is essential for the activation, maturation, and survival of auto-reactive B cells.
Ianalumab is a first-in-class monoclonal antibody that acts as a BAFF receptor antagonist with a dual mechanism:
- Signal Blockade: It interrupts survival and activation signals within B cells.
- Cell Depletion: It induces potent B-cell depletion via antibody-dependent cell-mediated cytotoxicity (ADCC).
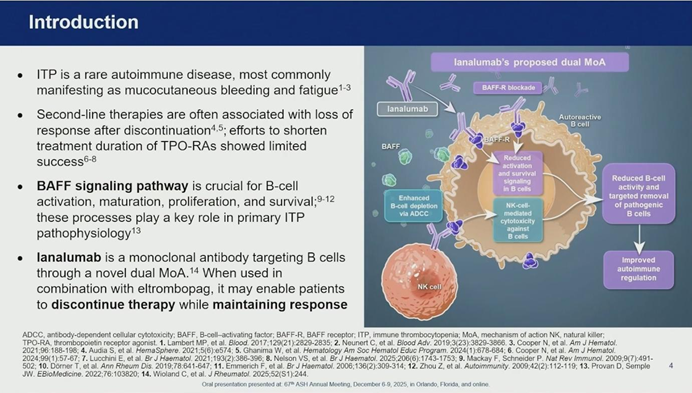
The rationale behind VAYHIT 2 was that introducing Ianalumab early in the treatment course (Second-Line) alongside a TPO-RA could induce durable remission, potentially freeing patients from the burden of chronic maintenance therapy.
02. Study Design: A Focus on Second-Line Treatment
The VAYHIT 2 study enrolled 152 adult patients with primary ITP who had failed first-line corticosteroids, had platelet counts <30,000/μL, and were naive to other second-line therapies.
Treatment Protocol:
Patients were randomized (1:1:1) to receive Eltrombopag combined with either:
- Ianalumab 3 mg/kg
- Ianalumab 9 mg/kg
- Placebo
The regimen consisted of monthly infusions for 16 weeks (4 doses). Subsequently, patients meeting specific criteria began tapering Eltrombopag, with the goal of discontinuing the TPO-RA completely by week 24.
Novel Primary Endpoint:
The study utilized a novel endpoint: Time to Treatment Failure (TTF). Failure was defined as platelet counts dropping below 30,000/μL, the need for rescue/new therapy, inability to taper Eltrombopag, or death.
03. Efficacy Analysis: Significant Prolongation of Disease Control
The study met its primary endpoint, demonstrating that Ianalumab significantly stabilizes disease control compared to standard of care alone.
- Time to Treatment Failure (TTF): Both Ianalumab dosages showed superior efficacy over placebo.
- Placebo Group: Median TTF was only 4.7 months.
- Ianalumab 9 mg/kg: Median TTF was extended to 13 months (HR 0.55).
- Ianalumab 3 mg/kg: Median TTF was not reached (HR 0.58).
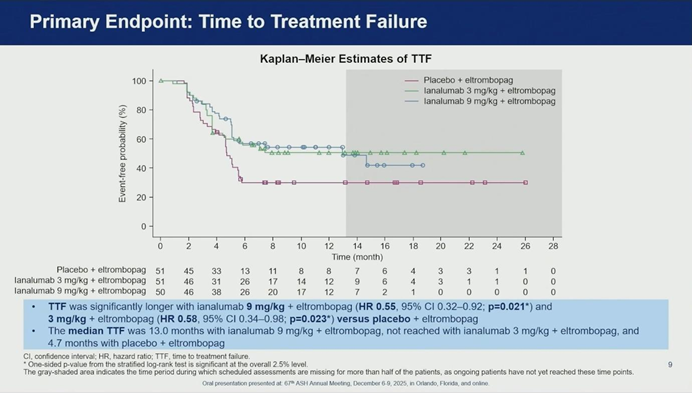
- Stable Response at 6 Months (SR6): The high-dose group (9 mg/kg) also met the key secondary endpoint, with 62% of patients achieving a stable response (maintaining platelets ≥50,000/μL while tapering TPO-RA) compared to only 39% in the placebo group.
Furthermore, patients in the Ianalumab arms experienced fewer bleeding events and reported greater improvements in fatigue scores (Quality of Life) post-tapering compared to the placebo group.
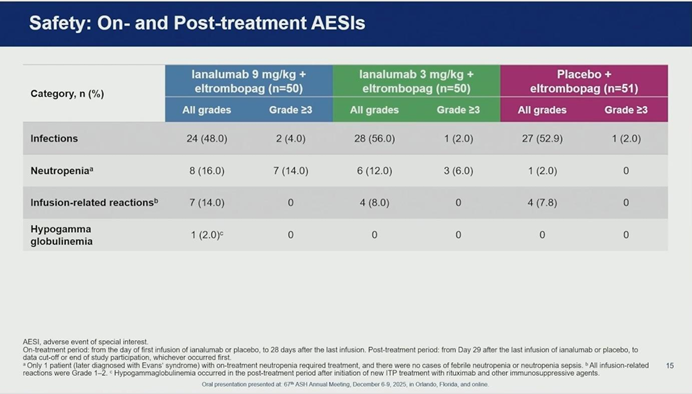
04. Safety Profile: No Increased Infection Risk
A critical concern with B-cell targeting therapies is immunosuppression. However, Dr. Al-Samkari reported that Ianalumab was well-tolerated with a safety profile consistent with previous data.
- Infections: Importantly, the incidence and severity of infections were similar between the Ianalumab and placebo groups.
- Adverse Events: Most adverse events were consistent across all arms. While neutropenia was more frequent in the Ianalumab groups, it was transient and did not require intervention. Infusion-related reactions were mild to moderate.
- Immunoglobulins: The impact on IgG levels was minimal, with only one case of hypogammaglobulinemia observed in the context of subsequent immunosuppressive therapy.
05. Mechanism Matters: Why Ianalumab is Not Rituximab
During the Q&A session, Dr. Al-Samkari addressed why Ianalumab succeeds where Rituximab (anti-CD20) often fails to provide long-term control. He outlined three fundamental differences:
- Potency: Ianalumab is glycoengineered for far superior B-cell depletion compared to Rituximab.
- Soluble BAFF Dynamics: Rituximab treatment causes a spike in soluble BAFF levels, which paradoxically supports the survival of long-lived plasma cells and disease persistence. Ianalumab, by blocking the BAFF receptor, prevents this negative feedback loop.
- Dual Action: Ianalumab blocks maturation signals that Rituximab cannot target.
Conclusion
The VAYHIT 2 study results present compelling evidence that adding Ianalumab to Eltrombopag in the second-line setting significantly improves outcomes for ITP patients. By attacking the disease pathology through the BAFF pathway, this regimen offers a higher probability of sustaining safe platelet counts without the need for chronic drug exposure. With ongoing trials in first-line (VAYHIT 1) and later-line (VAYHIT 3) settings, Ianalumab is poised to reshape the ITP treatment landscape.