
Editor's note : Recently, Nature Communications (IF=14.7) published an article entitled "Nivolumab plus anlotinib hydrochloride in advanced gastric adenocarcinoma and esophageal squamous cell carcinoma: the phase II OASIS trial" by Dr. Tianshu Liu of Zhongshan Hospital. The phase II OASIS trial (NCT04503967) investigated the combination of nivolumab (an anti-PD-1 antibody) and anlotinib hydrochloride (a multi-target tyrosine kinase inhibitor) for advanced gastric adenocarcinoma (GAC) and esophageal squamous cell carcinoma (ESCC). Conducted at Zhongshan Hospital, Fudan University, this trial aimed to assess the safety and efficacy of a chemotherapy-free regimen for patients who had progressed beyond first-line treatments. The study spanned from December 2020 to September 2022, with a median follow-up of 15 months.Study Design and Participants
The trial enrolled 48 participants, including 45 patients with GAC and 3 with ESCC. The therapy involved administering nivolumab 360 mg every three weeks alongside anlotinib hydrochloride 12 mg daily for two weeks per cycle. The primary endpoint was the overall response rate (ORR), while secondary endpoints included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and safety.
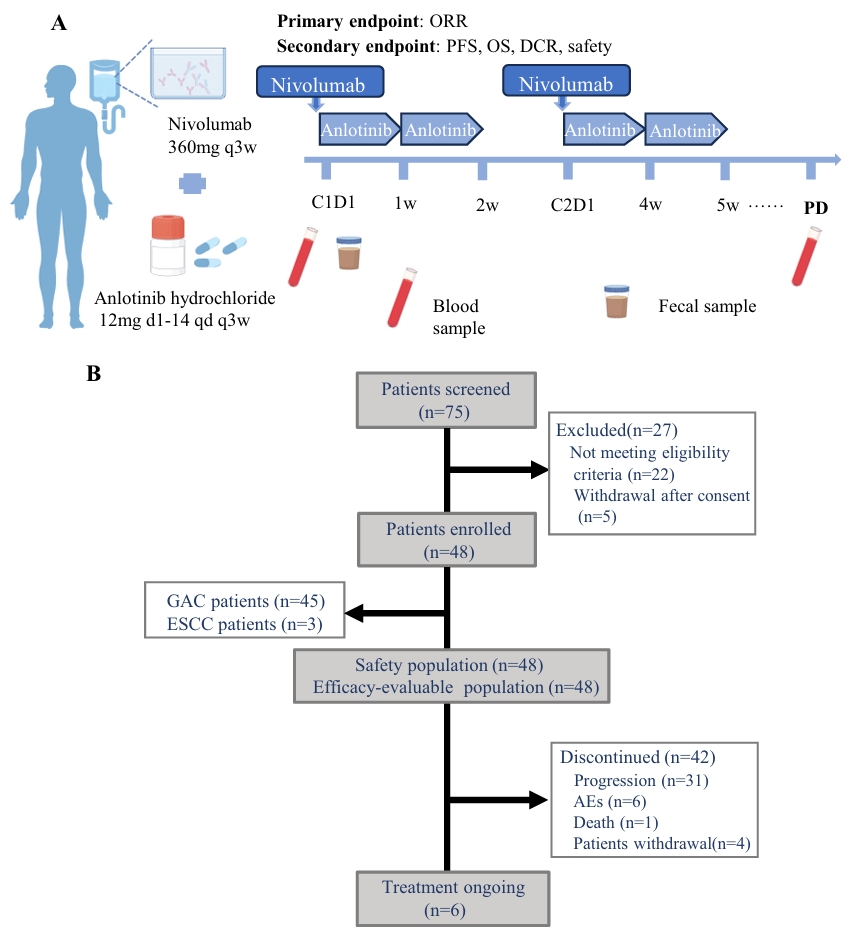
- Panel A: A schematic overview of the therapeutic regimen, showcasing the administration schedule of nivolumab and anlotinib hydrochloride, with sampling points for blood and fecal specimens.
- Panel B: Clinical trial profile illustrating patient flow, including the total patients screened (n=75), those enrolled (n=48), exclusions (n=27), and reasons for discontinuation (e.g., disease progression, adverse events, withdrawals). It highlights the safety and efficacy-evaluable populations, with key outcomes like ORR, PFS, OS, and DCR
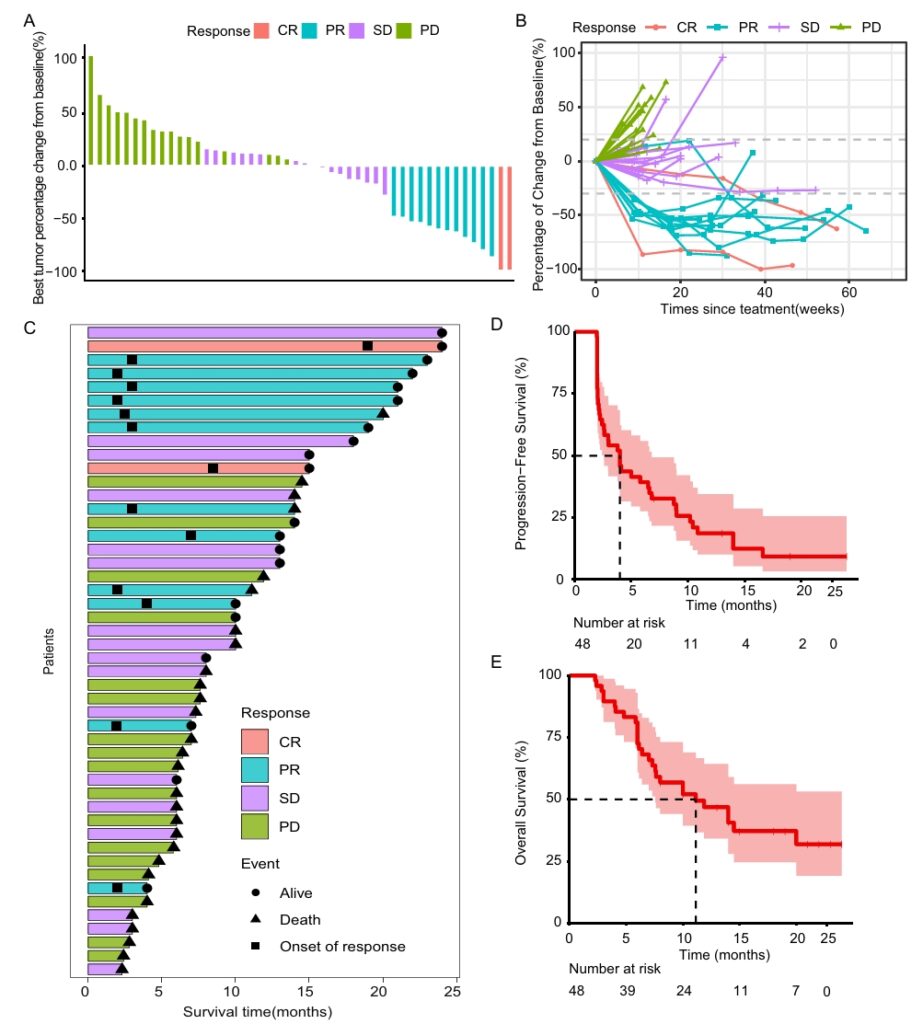
The combination therapy demonstrated promising efficacy. The ORR was 29.2% (95% CI: 17.0–44.1), with a DCR of 64.6% (95% CI: 49.5–77.8). The median PFS was 4.0 months (95% CI: 2.6–5.4), and the median OS was 11.1 months (95% CI: 5.7–16.5). Subgroup analysis revealed that second-line patients achieved an ORR of 32.3% and a median OS of 14.5 months, while third-line or beyond patients showed an ORR of 23.5% and a median OS of 7.6 months. These results underscore the potential of this combination for advanced-stage cancers.
This figure includes a waterfall plot showcasing the best percentage change in target lesion diameter from baseline within the efficacy-evaluable population (n=48). It also features spider and swimmer plots of tumor burden changes, treatment durations, and Kaplan-Meier curves for PFS (median: 4.0 months) and OS (median: 11.1 months). Shaded areas represent 95% confidence intervals.
Safety Profile
The safety profile was manageable, with treatment-related adverse events (TRAEs) occurring in 95.8% of patients. Common TRAEs included hypertension (16.7%), hypothyroidism (18.8%), and liver dysfunction (12.5%). Grade 3–4 TRAEs were observed in 16.7% of participants, involving conditions like hypothyroidism, rash, and intestinal obstruction or perforation. Importantly, there were no fatal adverse events, and the majority of side effects were mild to moderate.
Biomarker Insights
Exploratory biomarker analyses provided additional insights. Genomic and ctDNA profiling revealed frequent mutations in genes such as TP53, NCOR2, and MUC16, with lower pretreatment variant allele frequency (VAF) in ctDNA linked to improved survival outcomes. Immune profiling identified elevated levels of CD8+ T cells and CD68+ macrophages in responders, indicating a robust antitumor immune response. Responders also exhibited lower levels of immunosuppressive proteins, such as IL-6, IL-8, and CXCL1. Gut microbiome analysis further revealed an increased abundance of beneficial bacteria like Faecalibacterium and Parabacteroides in responders, highlighting a potential role of gut health in therapeutic efficacy.
Discussion
The combination of nivolumab and anlotinib displayed significant benefits, particularly as a second-line therapy, with favorable survival outcomes and a tolerable safety profile. These findings are consistent with prior studies combining VEGFR inhibitors and immune checkpoint inhibitors, but with a notably lower incidence of severe adverse events. However, limitations of the study include its small sample size, especially in the ESCC cohort, and its single-arm design, which restricts comparisons with other treatments.
Conclusion
This trial demonstrates that nivolumab combined with anlotinib is a promising treatment option for advanced GAC and ESCC. With manageable toxicity and meaningful efficacy, the therapy holds potential for broader application. Future multi-center trials with larger and more diverse populations are warranted to validate these results and refine biomarker-based strategies for patient selection.


