
Editor’s Note: The 65th American Society of Hematology (ASH) Annual Meeting was held in San Diego, California, from December 9 to 12, 2023. ASH Annual Meeting is the largest and most comprehensive international event covering both basic and clinical research in hematlogy. This year, the Hematology Department at West China Hospital, Sichuan University, presented two oral reports, and we delve into these studies:
#1
Short-Term Blinatumomab As a Bridge Therapy for Hematopoietic Stem Cell Transplantation in B-Cell Acute Lymphoblastic Leukemia with Low Leukemia Burden
Chinese Title: 短期倍利妥单抗桥接造血干细胞移植治疗低白血病负荷的急性B淋巴细胞白血病
Abstract Code: ORAL#0234
Presenter: Jie Ji
Corresponding Author: Jie Ji
Research Background
In patients with B-cell acute lymphoblastic leukemia (B-ALL), the impact of pre-transplant leukemia burden on relapse and long-term survival has been well established. Blinatumomab is a bispecific T-cell engaging antibody with significant efficacy in relapsed B-ALL. However, the standard dosage of blinatumomab (28 μg/day, continuous infusion for 28 days) is primarily established in relapsed B-ALL. Therefore, for patients with low tumor burden, the standard dosage may exceed the actual required dose, potentially leading to increased drug toxicity, delayed transplantation, and unnecessary economic expenditures.
Research Methods
This study included B-ALL patients with low tumor burden (bone marrow blasts ≤10%) planning to undergo allogeneic hematopoietic stem cell transplantation (Allo-SCT). Tumor burden was assessed using multiparameter flow cytometry or polymerase chain reaction (PCR) quantification targeting specific genes. Blinatumomab treatment started at an initial dose of 8 μg/day, gradually increased to 28 μg/day within 5-10 days, with a total dose of 175 μg. Bone marrow reassessment was performed after blinatumomab treatment, followed by prompt Allo-SCT. All patients underwent myeloablative conditioning (cyclophosphamide, fludarabine, cytarabine, and idarubicin). Peripheral stem cell donors included HLA-matched sibling donors (MSD), HLA-matched unrelated donors (MUD), or haploidentical donors (HID). Post-transplantation acute graft-versus-host disease (aGVHD) prophylaxis included post-transplant cyclophosphamide (PTCy) and cyclosporine. HID transplant recipients received mycophenolate mofetil from day +5 to +35 post-transplant. Patients were followed up at +1, +2, +3, +4, +6, +9, +12, +18, and +24 months after transplantation.
Research Results
The study included 20 patients, comprising 7 males and 13 females, with an age range of 15 to 58 years and a median age of 35.5 years. Patient and disease characteristics are summarized in Table 1. During blinatumomab treatment, 8 patients (40%) experienced grade 1 cytokine release syndrome (CRS), and 1 patient (5%) experienced grade 2 CRS; no grade 3 or higher CRS was observed. After blinatumomab treatment, all patients achieved complete remission (CR) and minimal residual disease (MRD) negativity by flow cytometry. Among 11 Philadelphia chromosome-positive ALL patients, 5 did not achieve MRD negativity by PCR. Four patients received MSD transplants, 2 received MUD transplants, and 14 received HID transplants, with a median time from blinatumomab treatment to transplantation initiation of 5.5 days (range: 1-29 days). Median neutrophil engraftment occurred at day 12 (range: 9-17 days), and platelet engraftment at day 13 (range: 9-28 days). The median follow-up time for all patients was 10.2 months (range: 2-21.7 months). On day 30 post-transplantation, all patients achieved CR and MRD negativity, with complete donor chimerism. The rates of grades II-IV acute GVHD and III-IV acute GVHD were 10.0% and 5.3%, respectively (Figure 1A). One patient died at 7.2 months post-transplantation due to COVID-19-related pulmonary aspergillosis. Among the 18 evaluable patients, the estimated 1-year cumulative incidence of chronic GVHD was 44.4%, with no severe chronic GVHD observed (Figure 1B). As of the last follow-up on July 31, 2023, no patients experienced relapse. The estimated 1-year progression-free survival (PFS) and overall survival (OS) were both 90% (Figure 1C).
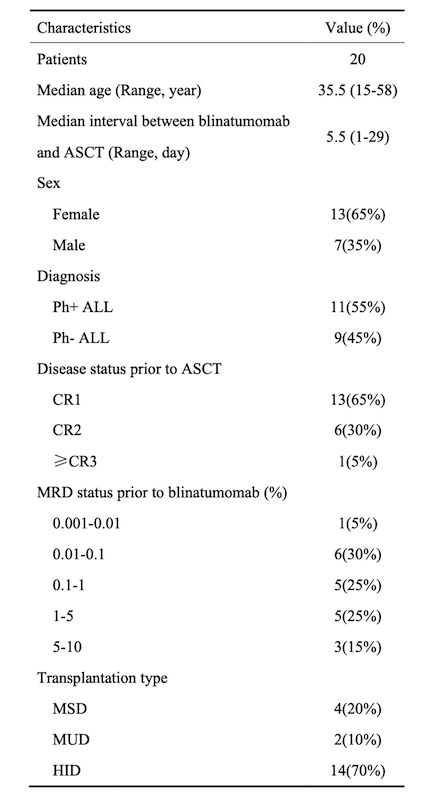
Table 1. Patient and Disease Characteristics
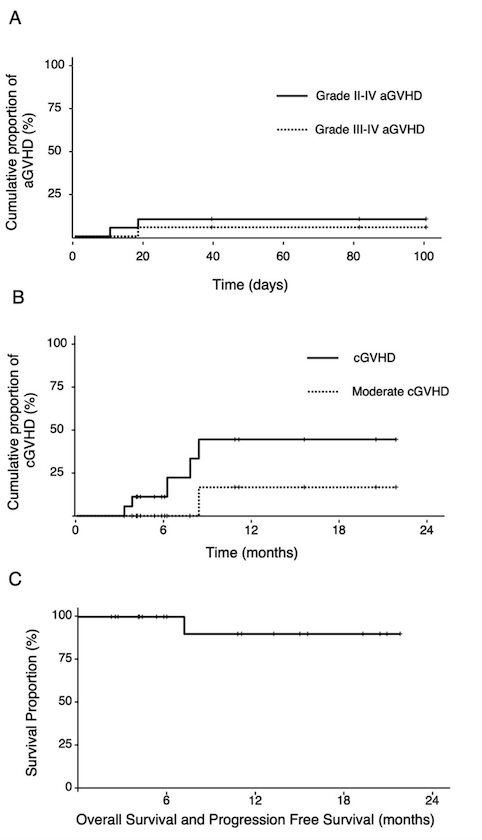
Figure 1. Cumulative incidence of acute GVHD, chronic GVHD, overall survival, and progression-free survival in patients.
Research Conclusion
For adults with low tumor burden B-ALL, short-term blinatumomab bridging therapy before Allo-SCT demonstrates satisfactory efficacy in disease control and patient survival.

Expert Commentary
Professor Ting Liu: In patients with acute B-cell lymphoblastic leukemia (B-ALL), pre-transplant minimal residual disease (MRD) increases the risk of post-transplant relapse, and conventional therapy struggles to further clear MRD, affecting long-term post-transplant survival. Blinatumomab is a bispecific T-cell engaging antibody with proven efficacy in both relapsed B-ALL and MRD clearance. This study, targeting B-ALL patients with low tumor burden, utilized short-term, low-dose blinatumomab bridging therapy before allogeneic hematopoietic stem cell transplantation. Patients rapidly achieved hematological remission and MRD clearance after blinatumomab treatment. The 1-year progression-free survival (PFS) and overall survival (OS) were both 90%, demonstrating satisfactory efficacy of short-term blinatumomab bridging therapy before allogeneic hematopoietic stem cell transplantation in disease control and patient survival. This provides a safe, effective, and economical treatment option for B-ALL patients with low tumor burden.
#2
Phase I/II Study of Reduced Dosing of Post-Transplantation Cyclophosphamide (PTCy) after HLA-Identical Peripheral Blood Hematopoietic Stem Cell Transplantation
Chinese Title: HLA全相合外周血造血干细胞移植后降低剂量的移植后环磷酰胺(PTCy)的I/II期临床研究
Abstract Code: ORAL#0650
Presenter: Hang Zhang
Corresponding Author: Jie Ji
Research Background
Post-transplantation application of cyclophosphamide (PTCy) has become the standard protocol for preventing acute graft-versus-host disease (aGVHD) in HLA-haploidentical bone marrow transplantation. Previous studies have shown that a reduced dose regimen is feasible and effective compared to the standard dose (50 mg/kg, post-transplantation day +3 and +4). However, in HLA-matched peripheral blood stem cell transplantation (PBSCT), the optimal dose of PTCy for aGVHD prevention remains unclear. This study aims to explore the efficacy and safety of reduced-dose PTCy in patients undergoing HLA-identical PBSCT.
Research Methods
This prospective, single-center, phase I/II clinical study included patients planning to undergo HLA-matched PBSCT. aGVHD prophylaxis involved administering PTCy on post-transplantation days +3 and +4, with cyclosporine starting on day +5. In the phase I trial, the initial dose was 50 mg/kg on days +3 and +4 (dose level 1, DL1). Subsequently, a 3+3 dose de-escalation design was implemented, with the following dose levels: DL2, 50 mg/kg on day +3 and 25 mg/kg on day +4; DL3, 25 mg/kg on both days +3 and +4. Dose-limiting toxicity during dose de-escalation was defined as grade III-IV aGVHD within the first 100 days post-transplantation.
The phase II study constituted an extension cohort based on the optimal dose determined in the phase I study. aGVHD grading utilized the Mount Sinai Acute GVHD International Consortium (MAGIC) standard, and chronic GVHD (cGVHD) assessment followed the latest National Institutes of Health consensus. Close patient follow-ups were conducted at +1, +2, +3, +4, +6, +9, +12, +18, and +24 months post-transplantation.
Research Results
The first phase included 11 patients (5 in DL1, 3 in DL2, and 3 in DL3), with no patients experiencing III-IV aGVHD. DL3 was selected as the PTCy dose for the second phase, involving a total of 18 patients. Of the 29 patients (17 females, 12 males) included in the study, with a median age of 39 years (range: 17-65 years) and a median follow-up time of 12.7 months (range: 2.2-22.1 months), all achieved complete remission (CR) and MRD clearance by day 30 post-transplantation. No patients experienced III-IV aGVHD within the first 100 days post-transplantation.
There were no significant differences in neutrophil and platelet engraftment times among the three groups (Figure 1A, B). In the 21 patients receiving DL3 PTCy, the cumulative incidence of grades II-IV aGVHD at day +100 was 28.6%, with no significant differences between the three dose groups (Figure 1C). In the DL3 group, one patient developed grade IV intestinal aGVHD on day +141 and died one month later. The estimated 1-year cumulative incidence rates of hemorrhagic cystitis (HC) and cytomegalovirus (CMV) reactivation were 19.3% and 19%, respectively (Figure 1D). Five patients, all in the DL3 group, experienced relapse, with a median relapse time of 6.7 months and a 1-year cumulative incidence rate of 10.6%. During the study, five patients died (1 in DL1, 4 in DL3), with one death due to relapse, two to COVID-19-related pulmonary aspergillosis, one to sepsis, and one to grade IV aGVHD. The estimated 6-month cumulative non-relapse mortality rate was 9.5%, with no differences among the three groups (Figure 1E). Among the 20 evaluable patients, the estimated 1-year cumulative incidence rates of cGVHD and moderate cGVHD were 37.3% and 16%, respectively (Figure 1F), with no occurrence of severe cGVHD. The estimated 1-year progression-free survival (PFS) and overall survival (OS) were 61.2% and 80.4%, respectively (Figure 1G).
Table 1. Patient and Disease Characteristics
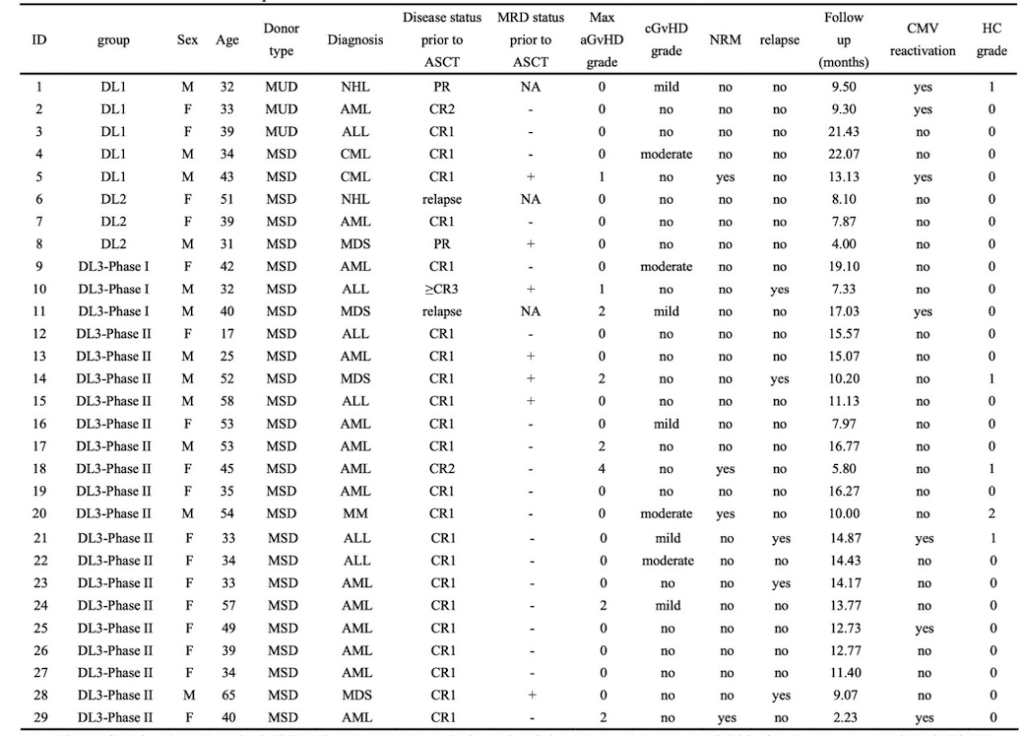
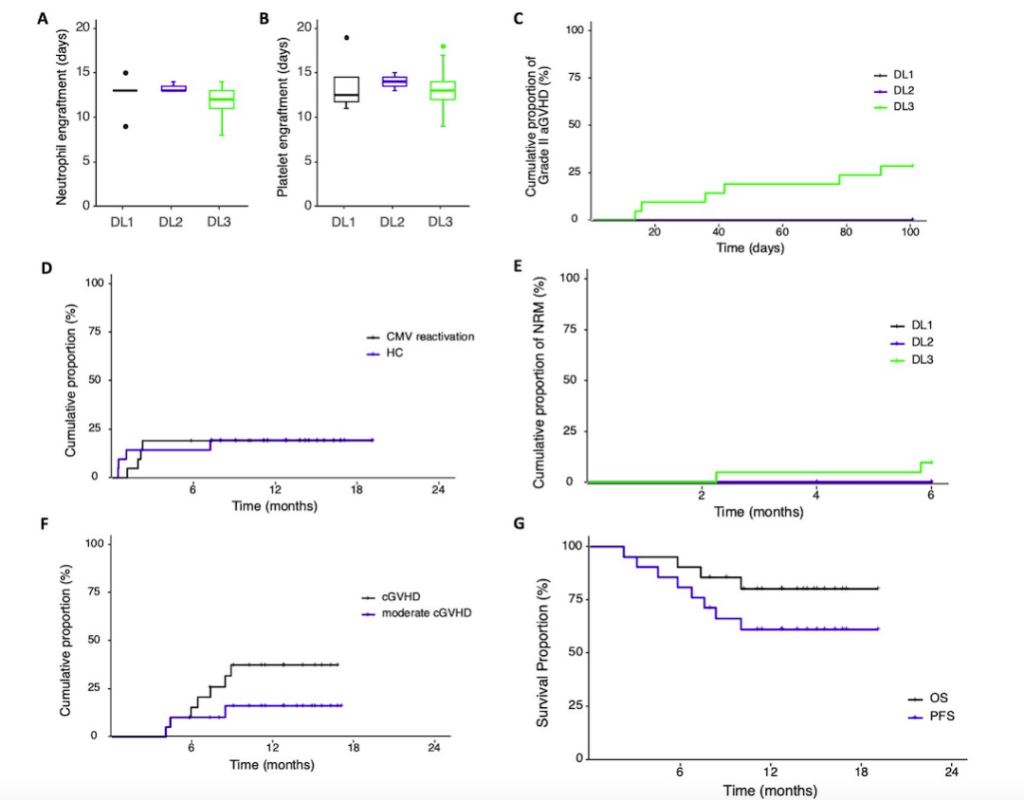
Figure 1. Cumulative incidence of engraftment times, CMV reactivation, hemorrhagic cystitis, acute GVHD, chronic GVHD, non-relapse mortality, overall survival, and progression-free survival in patients.
Research Conclusion
The results of this study confirm that PTCy at a dose of 25 mg/kg/day on days +3 and +4 is an effective and safe aGVHD prevention measure, comparable to the standard dose. Long-term follow-up and larger sample size controlled studies are needed to determine whether reduced-dose PTCy is superior to the standard dose.

Expert Commentary
Professor Ting Liu: Post-transplantation cyclophosphamide (PTCy) has become the standard protocol for preventing graft-versus-host disease (GvHD) in allogeneic hematopoietic stem cell transplantation. However, the current standard dose (50 mg/kg, post-transplantation day +3 and +4) is extrapolated from animal skin transplantation models, and there has been no rigorous dose exploration in clinical practice. Based on previous dose reduction studies in bone marrow transplantation, this study conducted a dose de-escalation investigation of PTCy in HLA-matched peripheral blood stem cell transplantation. The results confirmed that reduced-dose PTCy has similar efficacy in preventing GvHD compared to the standard dose, providing important guidance for the further optimization of PTCy clinical use.


