Editor’s Note:
Immune checkpoint inhibitors (ICIs) have been widely used in the clinical treatment of patients with advanced hepatocellular carcinoma (HCC), significantly improving their survival. While early-stage HCC can be cured with hepatectomy, up to 70% of patients may experience tumor recurrence within two years post-surgery. Therefore, the feasibility of perioperative ICI use to effectively inhibit tumor recurrence and further improve the postoperative survival rate of HCC patients is a subject that needs further exploration. From September 7th-9th, 2023, the global academic feast in the field of liver cancer—the 17th International Liver Cancer Association (ILCA) annual meeting was held grandly in Amsterdam, the capital of the Netherlands. Dr. Antonio D’Alessio from Imperial College London presented the PRIME-HCC study, a clinical trial of ICIs as a neoadjuvant treatment in unresectable HCC patients (Abstract number: O-13). This attracted wide attention from attendees, and Dr. Antonio D’Alessio was awarded the “Young Investigator” medal by the ILCA conference. Here’s a comprehensive report.
The PRIME-HCC study is a phase Ib clinical trial aiming to evaluate the safety and antitumor activity of the combination of Nivolumab (3 mg/kg, day 1 and day 22) and Ipilimumab (1 mg/kg, day 1), referred to as Nivo+Ipi, as neoadjuvant treatment in early-stage HCC patients before hepatectomy. Preliminary results of this study were reported at the American Society of Clinical Oncology (ASCO) annual meeting in 2023.
Primary endpoints of the study were treatment-related adverse events (trAE) and surgical delays. Secondary endpoints included the objective response rate (ORR) based on RECIST v1.1 evaluation and pathological response rate in resected specimens (refer to Figure 1).
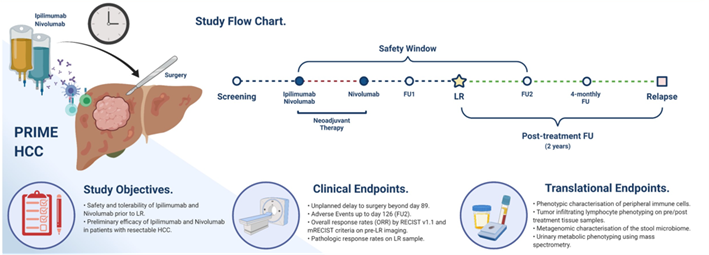
Figure 1. PRIME-HCC study flow chart
(from the ASCO 2023 conference poster).
According to preliminary results reported at the ASCO conference, 17 subjects were included. 73% of participants who received at least one treatment reported trAE of any grade (11/15), with 4 (27%) experiencing grade 2 trAEs, including hypothyroidism (n=2), diarrhea (n=1), and fatigue (n=1). One case (7%) had grade 3 ALT/AST elevation. With a median follow-up of 6.3 months, the ORR was 23% among 13 radiologically evaluable patients, with 2 partial responses and 1 complete response. Disease control rate was 92%. Out of 9 pathologically evaluable patients, 7 (78%) achieved a pathological response, including 2 (22%) with a complete pathological response. Preliminary conclusions suggest that Nivo+Ipi can be safely used as neoadjuvant treatment for HCC without delaying hepatectomy. Both radiological and pathological responses indicate that Nivo+Ipi has substantial antitumor efficacy.
At the ILCA conference, Dr. D’Alessio also incorporated translational endpoints, including multiplex immunohistochemistry (MIHC) analysis of tissue samples, quantifying circulating cell-free DNA (cfDNA) in plasma, and high-throughput sequencing analysis of gut microbiota correlation with treatment response. As of April 5, 2023, the study included 26 HCC patients, all of whom were Child-Pugh A, with 73% being male (n=19), 50% having cirrhosis (n=13), and 42% viral hepatitis (4 with HBV, 7 with HCV).
Results showed that 88% of patients (n=23) experienced any-grade adverse events (AE), with 23% (n=6) being grade 3, and no grade 4-5 events reported. 73% (n=19) reported trAEs of any grade, including 8% with grade 3 trAE (1 case of ALT/AST elevation, 1 of dyspnea). One patient was found to have cholangiocarcinoma (CCA) and was excluded from the efficacy analysis.
After a median follow-up of 14.5 months, 23 patients underwent radiological response assessment, and 22 underwent hepatectomy. The median time from ICI initiation to surgery was 2.4 (2.2-2.6) months. One patient had a surgical delay due to trAE (2-grade hypothyroidism), but remained stable. The ORR was 26% among all evaluable patients, with 5 partial responses and 1 complete response. The disease control rate was 96% (n=22), with one primary progressor having mixed HCC/CCA. Major pathological response (MPR), defined as ≥70% tumor reduction, was observed in 42% (n=8) of evaluable cases, with 32% (n=6) achieving pathological CR. No MPR patients reported disease recurrence. Compared to those without MPR, MPR patients had significantly improved relapse-free survival (RFS) (median RFS: 32.8 months vs. not reached, P=0.05).
MIHC analysis of paired pre- and post-ICI samples showed that pre-treatment biopsies from MPR patients had a significant enrichment of CD4+ and CD8+ cells in peritumoral areas, but no significant difference within tumors. Comparing baseline biopsy samples, tumor-infiltrating FOXp3+/CD4+ regulatory T cells (Tregs) significantly decreased in MPR patients, while only in non-MPR did peritumoral Tregs increase significantly post-ICI treatment. Furthermore, in patients with a high tumor burden, baseline plasma cfDNA levels were significantly higher and showed no significant change after ICI treatment. Analyses revealed that higher baseline cfDNA levels in non-MPR patients significantly correlated with worse RFS and overall survival. In baseline fecal samples, there was no difference in microbial diversity between responders and non-responders before or after ICI exposure. However, fecal samples from non-responder patients before treatment showed significant enrichment of the Alistipes and Collinsella genera.
In summary, the results of this study suggest that Nivo+Ipi as neoadjuvant treatment in HCC patients is feasible and tolerable, with positive radiological and pathological responses. Regarding translational endpoints, research into tissue, plasma, and gut microbiota highlighted tumor and host-related changes, suggesting immune cell infiltration, cfDNA, and gut microbiota composition as potential predictors of Nivo+Ipi’s antitumor efficacy. We also look forward to seeing substantive progress in subsequent postoperative follow-ups of the study, hoping that ICI as neoadjuvant treatment will prevent or delay tumor recurrence and improve the long-term prognosis of resectable HCC patients.
TAG: ILCA 2023, Commentary, HCC, Immunotherapy, Neoadjuvant Treatment

