At the Late-Breaking Abstract (LBA) session of the 2025 American Society of Hematology (ASH) Annual Meeting, Dr. María-Victoria Mateos, representing the research team, unveiled the highly anticipated results of the MAJEStec-3 study. This marks the first reported Phase III clinical trial of a BCMA-targeted bispecific antibody. Comparing the combination of Teclistamab and Daratumumab ("Tech-Dara") against standard-of-care regimens (Dara-Pd or Dara-Vd) in patients with relapsed/refractory multiple myeloma (RRMM), the study delivered historic results. The Tech-Dara combination achieved a Hazard Ratio (HR) for progression-free survival of 0.17—the most profound efficacy signal reported to date for BCMA-targeted therapies—signaling a potential "functional cure" for patients with early relapse.01. Study Design: Validating Immune Synergy in Early Relapse
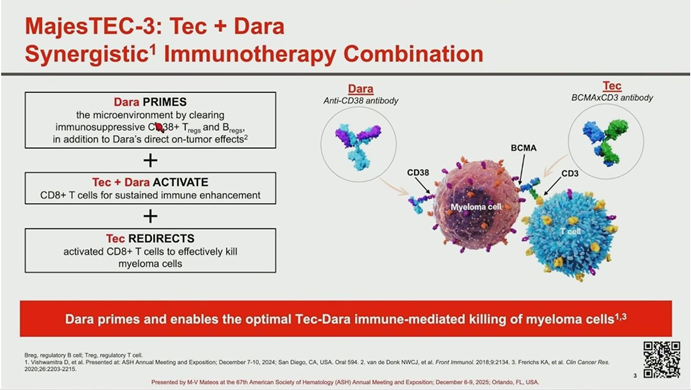
MAJEStec-3 is a global, randomized, open-label Phase III trial designed to test the hypothesis of immune synergy between the CD38-targeting antibody Daratumumab and the BCMA x CD3 bispecific antibody Teclistamab.
- Population: The study enrolled 587 RRMM patients who had received 1-3 prior lines of therapy. Crucially, while patients were refractory to lenalidomide and exposed to proteasome inhibitors, they were naive to both BCMA-targeted agents and Daratumumab.
- Regimen: Patients were randomized 1:1 to receive subcutaneous Tech-Dara or the investigator’s choice of Dara-Pd or Dara-Vd. A key quality-of-life feature for the Tech-Dara arm was the allowance for steroid-free treatment after Day 8 of Cycle 1.
- Demographics: Baseline characteristics reflected a real-world population (median age 64), with 35% of patients presenting with high-risk cytogenetics and 40% with soft-tissue plasmacytomas.
02. Primary Endpoint: A Historic HR of 0.17
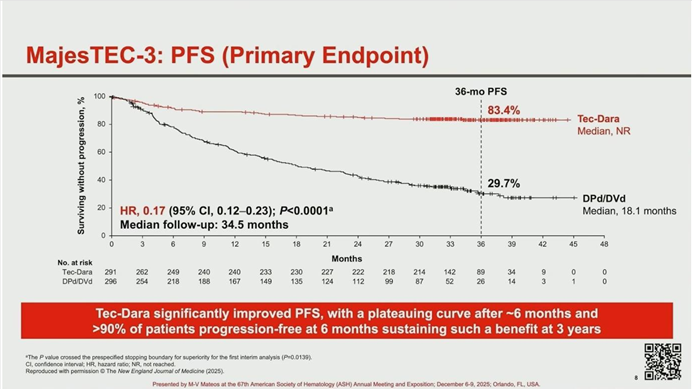
The primary endpoint of Progression-Free Survival (PFS) demonstrated overwhelming superiority for the immunotherapy combination.
- Risk Reduction: The Hazard Ratio for PFS was 0.17 (P < .0001), translating to an 83% reduction in the risk of disease progression or death compared to standard care.
- Survival Rates: At the 3-year mark, 83.4% of patients in the Tech-Dara arm remained alive and progression-free, compared to only 29.7% in the control arm.
- Plateau Effect: The PFS curve for the Tech-Dara arm showed a distinct plateau after month 6, suggesting that for a significant portion of patients, this combination may offer a functional cure. These benefits were consistent across all high-risk subgroups, including elderly patients and those with high tumor burden.
03. Depth of Response: Unprecedented MRD Negativity
Beyond survival duration, the depth of response induced by Tech-Dara was profound.
- Response Rates: The Overall Response Rate (ORR) was 89% for Tech-Dara versus 75.3% for the control. More importantly, 81.8% of Tech-Dara patients achieved a Complete Response or better (≥CR).
- MRD Status: In the Intention-to-Treat (ITT) population, the rate of Minimal Residual Disease (MRD) negativity (
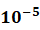
) was 58.4%, nearly triple that of the control arm (21.1%). Among evaluable patients, MRD negativity reached nearly 90%, confirming the regimen’s ability to eradicate deep-seated disease.
04. OS Benefit and Safety: Managing Early Infection Risk
While the study showed a significant Overall Survival (OS) benefit (3-year OS 83.3% vs. 65%; HR = 0.46), the safety analysis highlighted a critical need for infection management during the early treatment phase.
- Early Mortality: The survival curves crossed at month 10. This was attributed to early infection-related deaths in the Tech-Dara arm (12 deaths within the first 6 months), primarily involving COVID-19 and respiratory infections in patients who had not received immunoglobulin (IVIg) support.
- Protocol Amendment: Recognizing this signal, the protocol was amended to mandate prophylactic measures and IVIg use. Following this intervention, Grade 3-4 infection rates dropped significantly, and infection-related mortality after 6 months was limited to a single case.
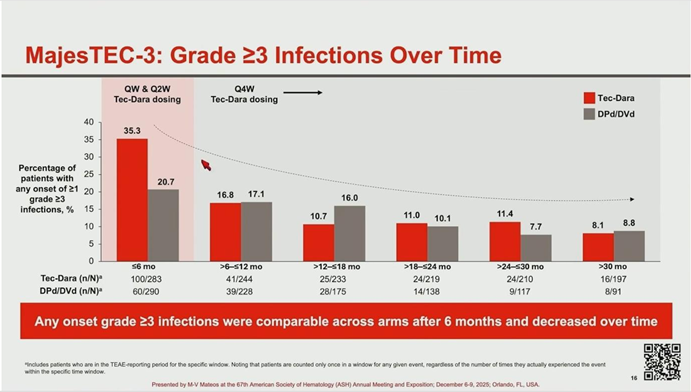
- Other Toxicities: Rates of CRS (60%, mostly low grade) and ICANS (1.1%) were consistent with known Teclistamab data and were manageable.
05. Conclusion: A New Standard of Care
Dr. María-Victoria Mateos concluded that the MAJEStec-3 study represents a major breakthrough in RRMM treatment. The “draining” of immunosuppressive cells by Daratumumab combined with the “redirected killing” of Teclistamab creates a potent synergistic effect.
With an HR of 0.17 and significant OS benefits, Tech-Dara is poised to become a new standard of care for lenalidomide-refractory patients who have received at least one prior line of therapy. The study also serves as a critical lesson in supportive care: with appropriate infection prophylaxis and IVIg replacement, this potent immunotherapy can be delivered safely, offering patients the prospect of deep, durable remission and potential functional cure.