
From January 26 to 28, 2024, the “Chinese Society of Clinical Oncology (CSCO) Leukemia Committee, Lymphoma Committee, and Myeloma Preparatory Committee Work Meeting and 2024 CSCO Blood, Lymphoma, and Myeloma Diseases Academic Conference” was held in Haikou. Many authoritative hematologists from home and abroad gathered to share their insights. Professor LanPing Xu from the Hematology Institute of Peking University People’s Hospital delivered an in-depth lecture on the topic of “Hematopoietic Stem Cell Transplantation for the Treatment of Severe Aplastic Anemia (SAA),” providing rich and fascinating content, summarized by “Oncology Outlook-Blood News.”
SAA is characterized by sudden onset, rapid progression, and a high mortality rate. Its first-line treatment options include immunosuppressive therapy (IST) and allogeneic hematopoietic stem cell transplantation (allo-HSCT). While immunosuppressive therapy is prone to relapse, with a low remission rate after relapse and a risk of evolving into clonal diseases such as Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML) in later stages, hematopoietic stem cell transplantation (HSCT) is currently the only definitive cure for aplastic anemia.
Pre-treatment Plan Stratification and Timing of Transplantation Make SAA Transplantation Safer
The transplantation treatment methods for SAA include matched sibling donor HSCT (MSD-HSCT), matched unrelated donor HSCT (MUD-HSCT), haploidentical HSCT (Haplo-HSCT), and cord blood transplantation (CB-HSCT).
The pre-treatment plans for allogeneic HSCT can be divided into myeloablative regimens, non-myeloablative regimens, and reduced-intensity regimens, with the latter two generally chosen for SAA transplants. Currently, the choice depends on the age and type of transplant: (1) For young SAA patients ≤30 years old with matched human leukocyte antigen (HLA), cyclophosphamide (Cy 200mg/kg) and rabbit ATG (rATG) are typically selected as the pre-treatment regimen; for HLA-matched SAA patients >30 years old, the pre-treatment regimen involves reduced dose Cy-ATG plus fludarabine (Flu); (2) MUD-HSCT for SAA patients commonly uses Flu/Cy/ATG±low-dose TBI as the pre-treatment regimen; (3) The pre-treatment regimen for cord blood transplantation also often adopts Flu/Cy/ATG±low-dose TBI; (4) The transplant schemes for treating SAA with haplo-HSCT mainly include a regimen based on granulocyte colony-stimulating factor (G-CSF), BuCy (busulfan and cyclophosphamide), (known as the “Beijing protocol”) and a post-transplant cyclophosphamide (PTCy) based scheme (known as the “Baltimore protocol”).
There are also studies on the pre-treatment schemes for older SAA patients undergoing transplantation. A retrospective study analyzed 499 patients aged ≥50 years with SAA between 2005 and 2016 from the Center for International Blood and Marrow Transplant Research (CIBMTR) and the European Society for Blood and Marrow Transplantation (EBMT). In this study, the MSD-HSCT group (n=275) used Cy-ATG or (+Flu) for pre-treatment, while the MUD-HSCT group used Cy+TBI+ATG or (+Flu) for pre-treatment. The results showed that, in SAA patients aged ≥50 years, the age at the time of transplantation was not associated with overall survival (OS) or the incidence of chronic graft-versus-host disease (cGVHD), but patients aged ≥65 years had a higher incidence of grade II-IV acute graft-versus-host disease (aGVHD) (see below for details).
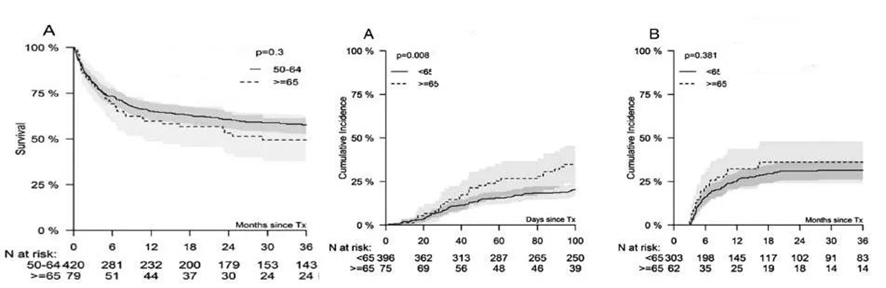
A retrospective study conducted in London and Italy involved 65 patients with SAA treated with matched related donor (MRD) or matched unrelated donor (MUD) transplants, using a pre-treatment regimen of fludarabine, low-dose cyclophosphamide, and alemtuzumab (FCC). The results indicated that the clinical outcomes of the FCC regimen were similar in patients aged ≥50 years and <50 years, suggesting that the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) is a more important consideration for the suitability of transplantation in elderly patients rather than age itself. Patients with an HCT-CI ≥3 had significantly shorter overall survival (OS) compared to others (as shown in the chart below).
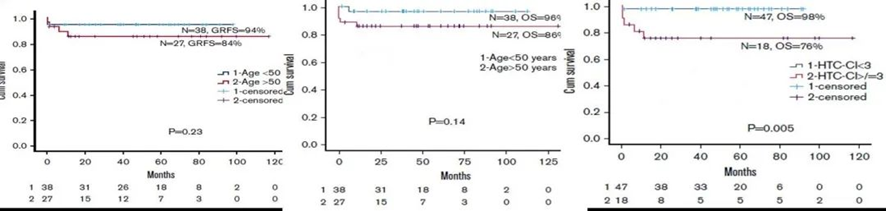
(Blood Adv. 2019 ,3(20):3070-3079)
In a single-center retrospective study in South Korea, a pre-treatment regimen of TNI-750 (total nodal irradiation of 750 cGy) plus ATG (antithymocyte globulin) was used for elderly patients with severe aplastic anemia (SAA) who also had severe comorbidities. This approach achieved favorable outcomes, with a 3-year overall survival (OS) rate of 100%, making it a viable and urgent treatment option.
Additionally, exploration has been conducted for pre-treatment regimens in haploidentical transplantation for patients at high risk of cardiac toxicity. A study by Professor Huang Xiaojun’s team published in Clinical Transplantation investigated reducing the dose of cyclophosphamide (Cy) from 200 mg/kg to 100 mg/kg and adding fludarabine (Flu) at 150 mg/m2, forming a BuCyLowFlu group. The results showed no difference in overall survival (OS) between the two regimens. However, the adjustment in the pre-treatment regimen could reduce cardiac toxicity associated with pre-treatment in haploidentical transplantation (as detailed in the chart below).
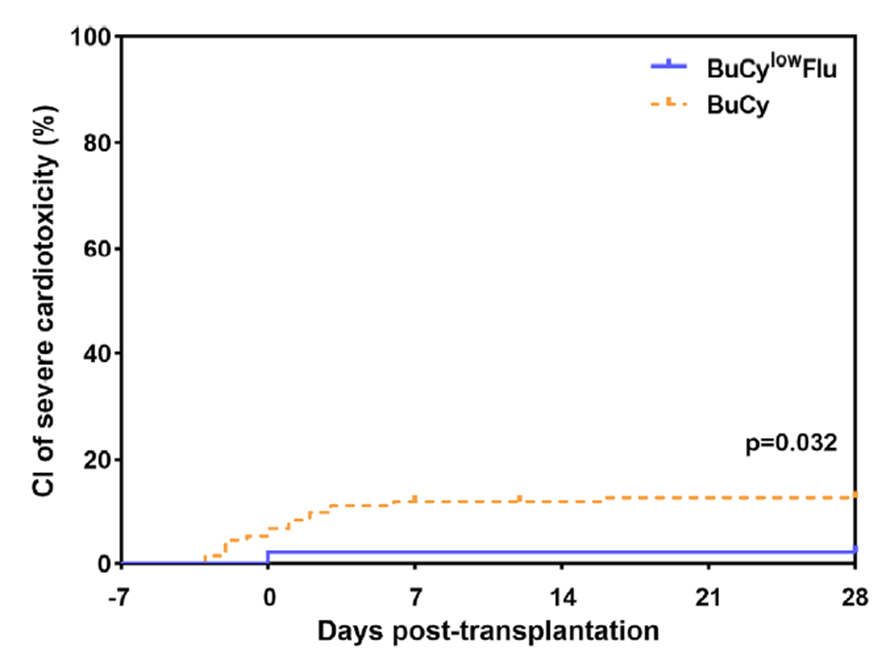
The occurrence of severe cardiac toxicity was significantly reduced in the BuCyLowFlu group.
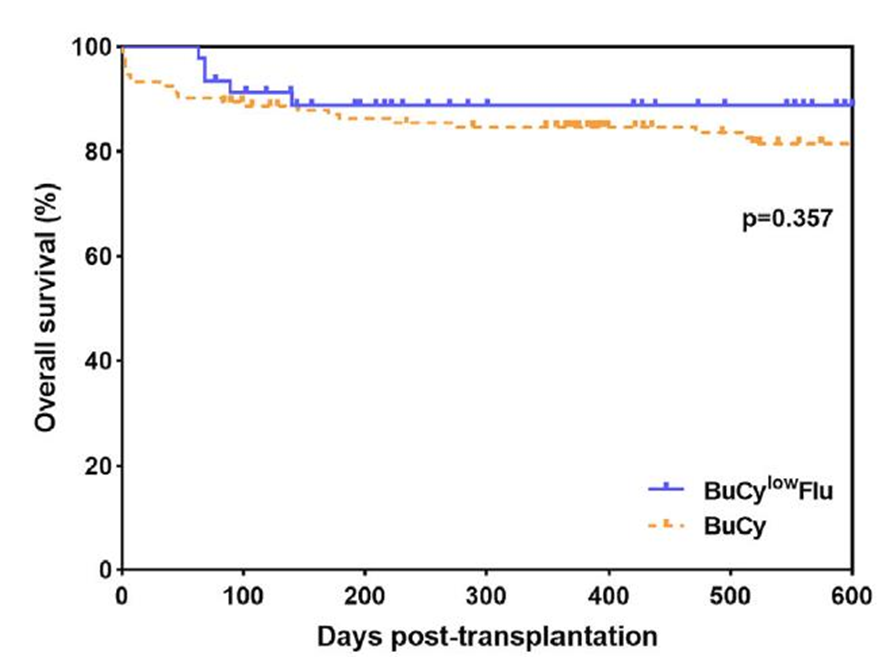
There was no difference in overall survival (OS) between the two pre-treatment regimens.(Huang XJ etal.Clinical Transplantation.2022)
The “Chinese Guidelines for the Diagnosis and Treatment of Aplastic Anemia (2022 Edition)” emphasize the following key points regarding the treatment of severe aplastic anemia (SAA):
1. First-Line Treatment Choice: When choosing first-line treatment, factors such as age, comorbidities, disease severity, Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), and other prognostic factors should be considered. If there are multiple favorable factors for a good prognosis with immunosuppressive therapy (IST), the use of IST combined with thrombopoietin receptor agonists (TPO-RA) is preferred as the first-line treatment. If there are multiple unfavorable prognostic factors for IST, and if conditions allow, hematopoietic stem cell transplantation (HSCT) should be chosen; the decision to choose TPO-RA+IST needs careful consideration of the benefits and risks.
2. Haploidentical HSCT (Haplo-HSCT): Haplo-HSCT can be considered as an additional treatment option for patients lacking a matched sibling donor (MSD) or matched unrelated donor (MUD). In experienced transplant centers, Haplo-HSCT can be used as a first-line treatment for patients lacking an MSD.
Additionally, the guidelines indicate that MSD-HSCT is the preferred treatment option for newly diagnosed SAA patients ≤50 years old. If there is no MSD available, both haploidentical transplantation and 10/10 fully matched unrelated donor (MUD) transplantation are viable options. Some experienced transplant centers in China have extended the age limit for first-line allo-HSCT treatment of SAA to ≤50 years and for second-line salvage treatment to 50–60 years, gaining some experience in this area. A specific treatment roadmap is illustrated below.
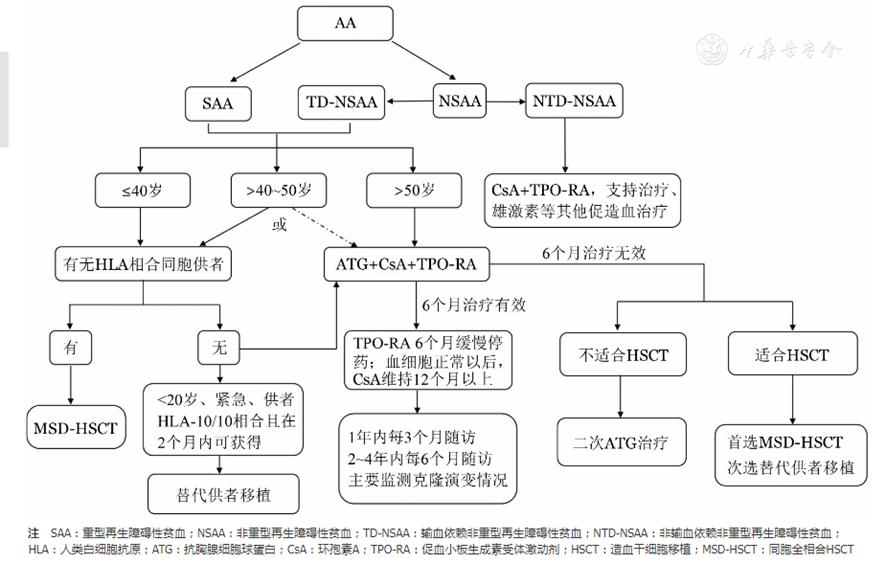
(Chinese Journal of Hematology, 2022, Volume 43, Issue 11, Pages 881-888)
Research analysis indicates that SAA patients aged 40 and above do not respond well to immunosuppressive therapy (IST) even when following guidelines. A study from the Netherlands involving 117 adult SAA patients treated with ATG+CsA as first-line therapy showed a 5-year overall survival (OS) rate of 77% and a 5-year treatment-free survival (TTT-FS) rate of 42% with IST (46% for patients ≤40 years and 33% for those >40 years), indicating that patients ≥40 years old have a lower TTT-FS after IST treatment.
Reports have shown that SAA patients over 40 can achieve better outcomes with transplantation. A study from China compared the efficacy of haploidentical transplantation (using the Beijing protocol, n=35), matched sibling donor (MSD) transplantation (n=38), and matched unrelated donor (MUD) transplantation (n=12) in patients aged ≥40 years with SAA, with a median follow-up of 17.6 months. The results indicated: (1) There was no statistical difference in 3-year OS and failure-free survival (FFS) among the groups, with an ECOG score ≥2 being the only independent factor affecting survival; (2) There was no statistical difference in grades 3-4 acute graft-versus-host disease (aGVHD) and moderate to severe chronic GVHD (cGVHD) among the groups.
Discussion on the Choice of Treatment for SAA
Elderly patients with SAA can also benefit from transplantation. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative method for SAA, capable of long-term hematopoietic reconstruction, avoiding clonal evolution, and its effectiveness is improving year by year. However, it should be noted that registry reports have shown that SAA patients over 40 not only have less effective outcomes with IST compared to younger patients but also fare worse in transplantation outcomes. Data from the European Society for Blood and Marrow Transplantation (EBMT) from 2005-2009 show that patients with SAA over 40 years old, whether receiving first-line matched sibling donor HSCT (MSD-HSCT), IST, or matched unrelated donor HSCT (MUD-HSCT), have significantly lower overall survival (OS) compared to SAA patients aged ≤20 and 21-40 years. This situation may be partly due to the delay in transplantation caused by current guidelines or consensus, and the effectiveness of transplantation is also related to the scale and experience of the transplant unit.
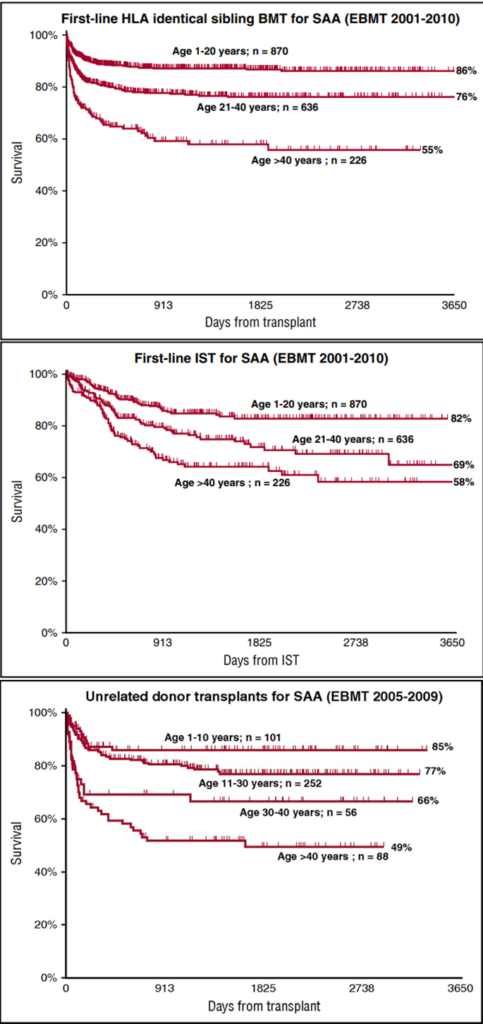
(Andrea Bacigalupo(Blood. 2017;129(11):1428-1436)
It’s important to note that delays in transplantation affect the efficacy of treatment across all age groups of SAA patients, including the elderly. A comprehensive analysis from the EBMT SAA Working Party published in Haematologica in 2023 showed that: (1) In a comparison of allogeneic hematopoietic stem cell transplantation (allo-HSCT) outcomes across all age groups of SAA patients, those over 40 years old had worse survival outcomes than those aged ≤20 and 21-40 years after receiving allo-HSCT; (2) Across all age groups, early transplantation (first-line transplantation, disease duration ≤6 months) had better outcomes than late transplantation (first-line transplantation, disease duration >6 months); the outcomes of unrelated donor (URD) transplants were similar to those of matched sibling donor (MSD) transplants (see details in the chart below).
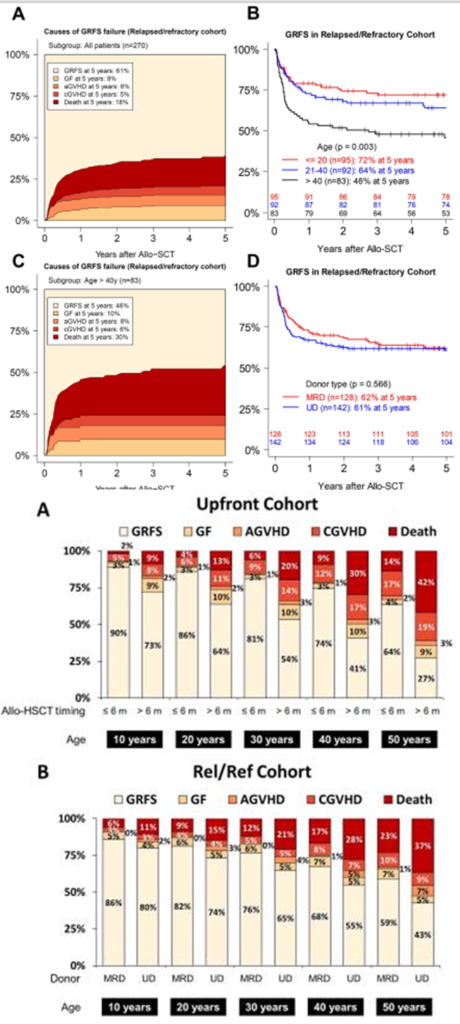
(Haematologica. 2023 Mar 23)
Identifying SAA patients, particularly those over 40 years old, who are unlikely to benefit from IST and prioritizing them for transplantation is crucial. A study published in the Chinese Journal of Hematology in 2022 summarized the predictive factors for the effectiveness of IST treatment, detailed in the chart below.
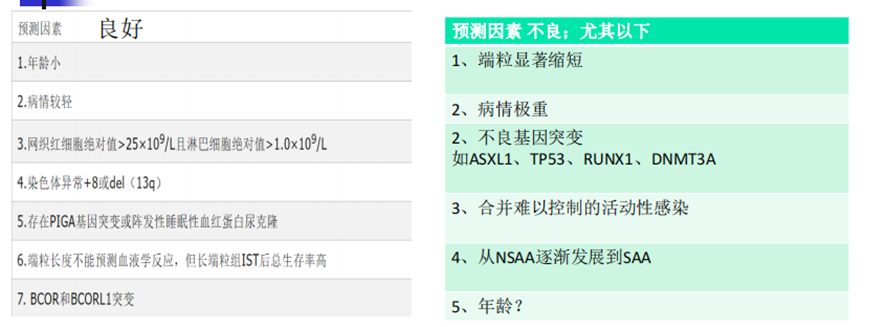
(Chinese Journal of Hematology, 2022, Volume 43, Issue 11, Pages 881-888)
Several studies have explored this topic, including a Phase II study led by Professor Daria V. Babushok and BA Patel, which utilized immunosuppressive therapy (IST) combined with eltrombopag for treatment. This study was published in the 2022 issue of the journal BLOOD. The results indicated that SAA patients with minimal residual bone marrow, older age, and who achieved partial remission (PR) after 6 months of treatment with eltrombopag had a high failure rate upon discontinuation of eltrombopag. These patients should be evaluated for transplantation possibilities as early as possible.
Additionally, other factors, such as HLA, are related to the efficacy of IST. A study published in BLOOD in 2021 showed that HLA-B*14:02, loss of HLA antigen expression (HLA-loss), and age ≥40 are associated factors for high-risk clonal evolution after IST treatment. (See details in the chart below).
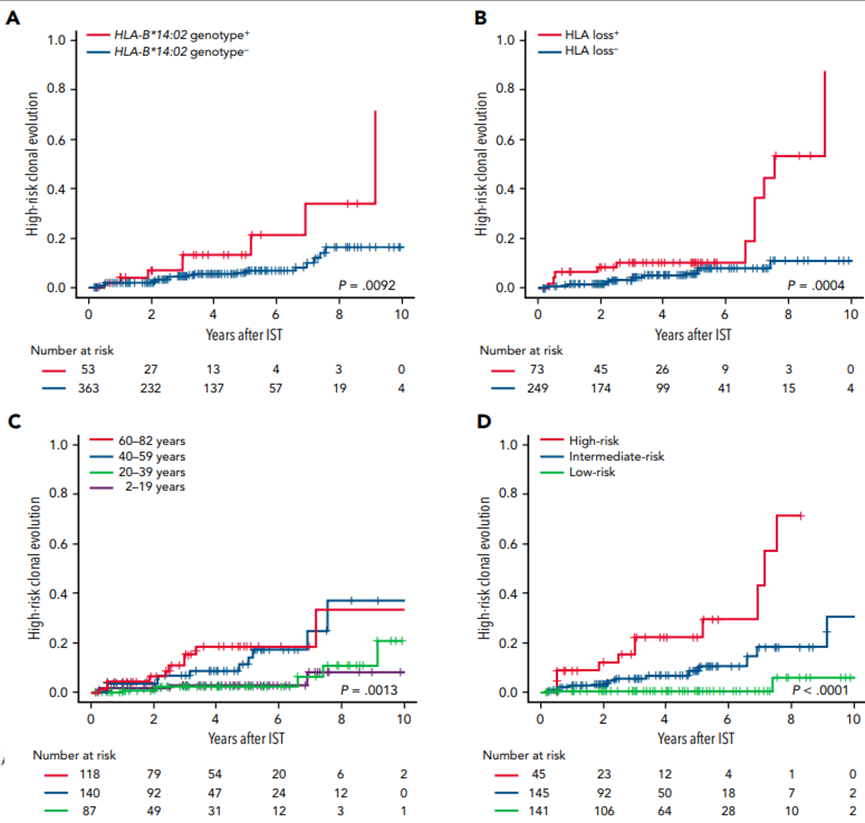
(BLOOD , 2021 ,138,(26):2799)
Several studies have explored this topic, among them, a Phase II study conducted by Professor Daria V. Babushok, Patel BA, and others, which utilized IST combined with Eltrombopag for treatment. This study was published in the BLOOD journal in 2022. The results indicated that SAA patients with minimal residual bone marrow, older age, and who achieved partial remission (PR) after 6 months of treatment have a high failure rate upon discontinuation of Eltrombopag (Elt). These patients should be evaluated for transplantation possibilities as soon as possible.
Additionally, other factors like HLA are also related to the effectiveness of IST. A study published in BLOOD in 2021 showed that HLA-B*14:02, HLA antigen expression loss (HLA-loss), and age ≥40 are factors associated with a high risk of clonal evolution after IST. (See chart below for details).
A study from China published in Frontiers in Immunology showed that: (1) HLA-B*15:18 and HLA-C*04:01 are associated with a good response to IST, while HLA-B*40:01 is associated with a poor response; (2) HLA A*01:01 and HLA-B*54:01 are associated with a high risk of high-risk clonal evolution (HI-CE), more commonly seen in very severe aplastic anemia (vSAA) (12.7% vs 0%); (3) HLA-DQ*03:03 and HLA-DR*09:01 are associated with high-risk clonal evolution and poor survival in patients aged ≥40 years.
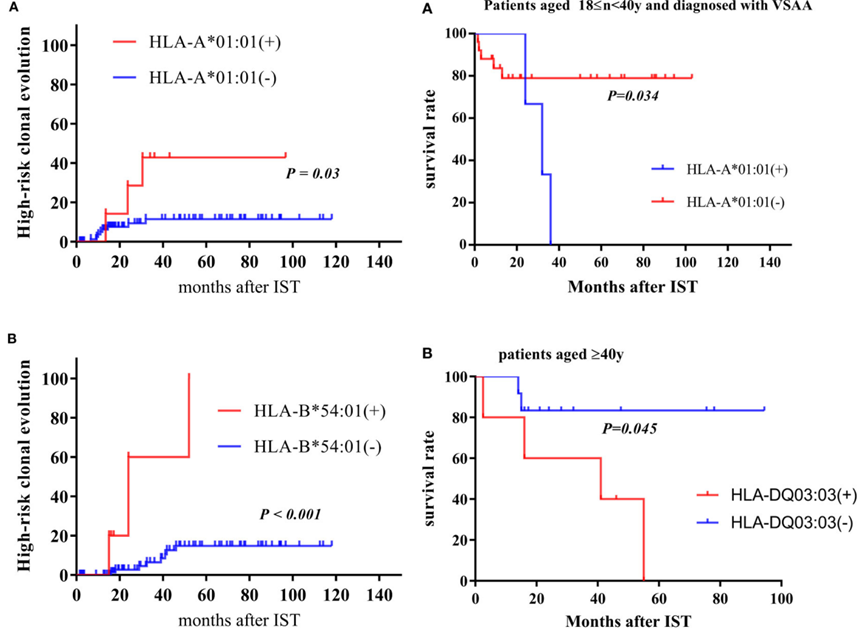
(Yizhou Zheng,et al. Frontiers in imunology 2023)
Be wary of inherited bone marrow failure syndromes (iBMFS) that onset in adulthood; transplantation is the preferred treatment for iBMF patients. Diagnosing iBMFS is highly challenging due to the poor accessibility of specialized tests such as chromosomal breakage studies, telomere length (TL) measurement, or genetic testing. Therefore, undiagnosed iBMFS poses a significant risk to patient treatment, as missing a diagnosis of iBMFS can lead to increased risks of organ involvement and malignancies. Patients with iBMFS typically do not respond to IST, making transplantation the preferred option.
A retrospective study published in 2023 by Zhejiang University of Chinese Medicine in the Annals of Hematology used whole-exome sequencing to identify FANC heterozygous germline mutations as a negative factor for the effectiveness of IST in Chinese patients aged ≤40 years, without affecting the efficacy of transplantation. New testing methods may aid in the selection of treatment strategies.
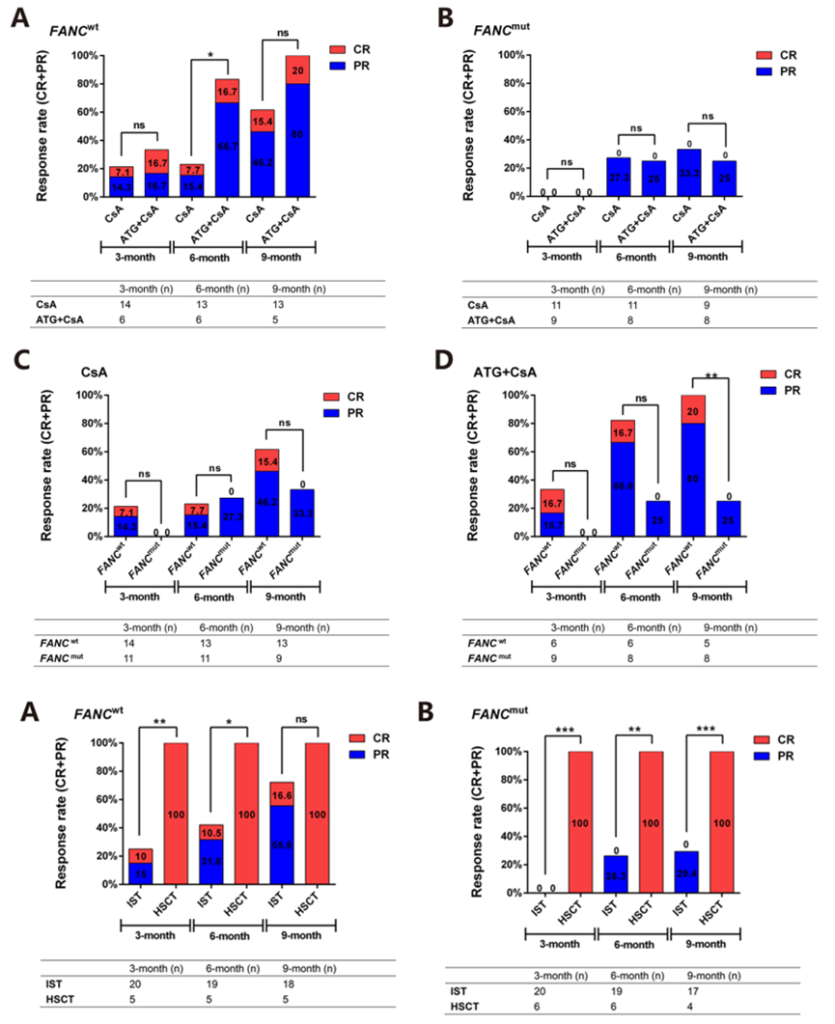
(Annals of Hematology (2023) 102:503–517)
In a study published in BLOOD in 2022 by Lisa J. McReynolds and others, genetic testing was used to identify “undiscovered iBMF” in patients with acquired SAA, which subsequently optimized transplantation efficacy. Compared to patients with acquired SAA, patients with “undiscovered iBMFS” exhibited poorer survival rates after hematopoietic cell transplantation (HCT) (HR 2.13; P=0.0004) and a higher rate of transplantation-related mortality. This suggests that the transplantation approach for iBMF patients needs to be personalized.
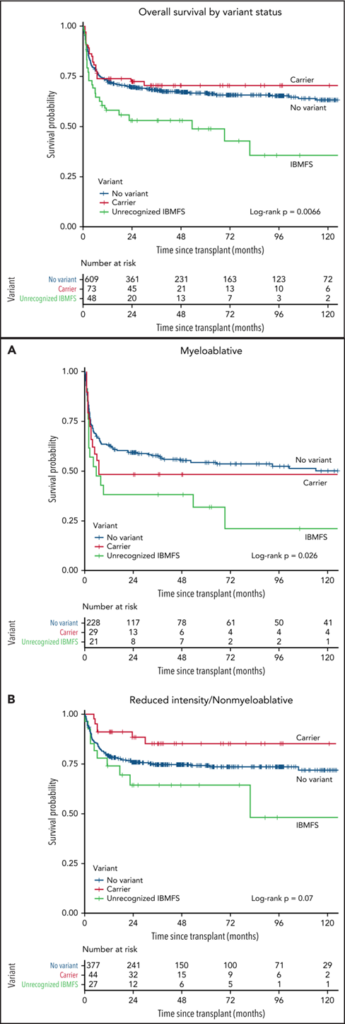
(Lisa J. McReynolds,et al.Blood . 2022 , 25;140(8):909-921.)
In summary, while age is an important basis for selecting treatment options for SAA, it is not the only one. Predicting the effectiveness of IST and transplantation separately to guide individual patients in choosing their treatment plan by weighing the benefit/risk ratio holds the promise of finding the best treatment path for each patient, thereby improving the overall prognosis for SAA patients.

Professor LanPing Xu
Professor LanPing Xu serves as the Deputy Director and Chief Physician of the Hematology Department at Peking University People’s Hospital, which is part of the National Clinical Research Center for Hematologic Diseases. She is also a doctoral supervisor. Professor Xu has held several prestigious positions, including being an Executive Director of the Cross-Strait Medicine and Health Exchange Association during its third and fourth terms, the Head of the Hematology Committee for its second and third terms, and the Head of the Expert Committee for the third term of the Beijing branch of the China Marrow Donor Program. She is also an active member of the Cell Research and Treatment Committee of the Chinese Research Hospital Association and the Clinical Microbiology and Infection Branch of the China International Exchange and Promotive Association for Medical and Health Care. Additionally, she has served on the Expert Committee for the eighth and ninth terms of the Chinese Hematopoietic Stem Cell Donor Database.Professor Xu is an editorial board member of several journals, including “Leukemia & Lymphoma,” “Chinese Journal of Geriatrics for Multiple Organ Diseases,” and “Chinese Journal of Hematology.” She has been listed twice as a recipient of the Second Prize of the National Science and Technology Progress Award in 2014 and 2017.Her main research focus is on the treatment of bone marrow failure diseases through hematopoietic stem cell transplantation. As a responsible author or first author, she has published over 50 SCI articles, co-authored more than 500 articles, and contributed to over ten textbooks or monographs.


