
With unceasing exploration, the grand event took place as scheduled. From January 26 to 28, 2024, the “Chinese Society of Clinical Oncology (CSCO) Leukemia Committee, Lymphoma Committee, and Myeloma Committee Work Meeting and the 2024 CSCO Academic Conference on Hematology, Lymphoma, and Myeloma” was grandly held in the beautiful city of Haikou. Renowned experts and scholars from home and abroad gathered to share and exchange on the latest advancements and hot topics in the field of hematologic malignancies. At the conference, Professor DeHui Zou from the Lymphoma Diagnosis and Treatment Center of the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences, presented on “Progress and Considerations in CAR-T Therapy for B-Cell Lymphomas,” sharing the latest research progress in the efficacy, therapeutic status, challenges faced, and optimization strategies for CAR-T treatment of Diffuse Large B-Cell Lymphoma (DLBCL). The following is a special summary.

CAR-T Therapy Efficacy—Further Validated, Demonstrating Curative Potential
In recent years, CAR-T cell therapy targeting CD19 has achieved significant success in the field of B-cell Non-Hodgkin Lymphoma (B-NHL). Currently, several anti-CD19 CAR-T cell therapies have been approved internationally for patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL) who have undergone ≥2 lines of prior therapy, including Axicabtagene Ciloleucel (Axi-cel), Tisagenlecleucel (Tis-cel), Lisocabtagene Maraleucel (Liso-cel), and Relmacabtagene Autoleucel (Relma-cel), with Axi-cel and Relma-cel being approved in China in June 2021 and September 2021, respectively.
Key clinical trials such as ZUMA-1, JULIET, and TRANSCEND NHL 001 have confirmed that Axi-cel, Tis-cel, and Liso-cel significantly improve the overall survival (OS) and progression-free survival (PFS) rates of DLBCL patients. The latest 6-year follow-up results of the ZUMA-1 study, presented at the 2023 American Society of Hematology (ASH) annual meeting, further demonstrated that 59 patients achieving the best complete response (bCR) with Axi-cel therapy had a 5-year complete response duration (DOCR) rate of 56.7%, with the 12-month and 24-month continuous CR patients having 5-year DOCR rates of 84.3% and 91.3%, respectively, showcasing curative potential. Similarly, the two-year follow-up results for Relma-cel, presented at the 2022 American Society of Clinical Oncology (ASCO) annual meeting, were also encouraging, with a median duration of response (DOR) of 20.3 months and a 2-year DOR rate of 40.3%. In the commercial dose group (100×10^6 dose group), the median DOR was 23.3 months, with a 2-year DOR rate of 56.18%.

6-year follow-up results of the ZUMA-1 study
Additionally, at the 2023 ASH annual meeting, results from two real-world studies (RWS) about Axi-cel were announced. One of these was from the U.S. Lymphoma CAR-T Consortium RWS, which showed that Axi-cel treatment for patients with relapsed/refractory (R/R) LBCL had a 5-year overall survival (OS) rate of 40.3%, confirming the consistency of Axi-cel’s real-world efficacy with that of clinical trials. Despite 43% of the patients not meeting the ZUMA-1 study criteria, 29% of patients still experienced ongoing remission at a median follow-up of 58 months. The other study was the first RWS conducted on a Chinese population, with interim analysis indicating that the median duration of response (DOR) for Axi-cel treatment in R/R NHL was 13.7 months; patients achieving the best complete response (bCR) did not reach median DOR, while those with the best response of partial remission (PR) had a median DOR of 4.7 months; the median progression-free survival (PFS) was 14.6 months; and the median OS was not reached, with an estimated 12-month OS rate of 80.4%. Also, no new adverse events were observed among the Chinese participants.
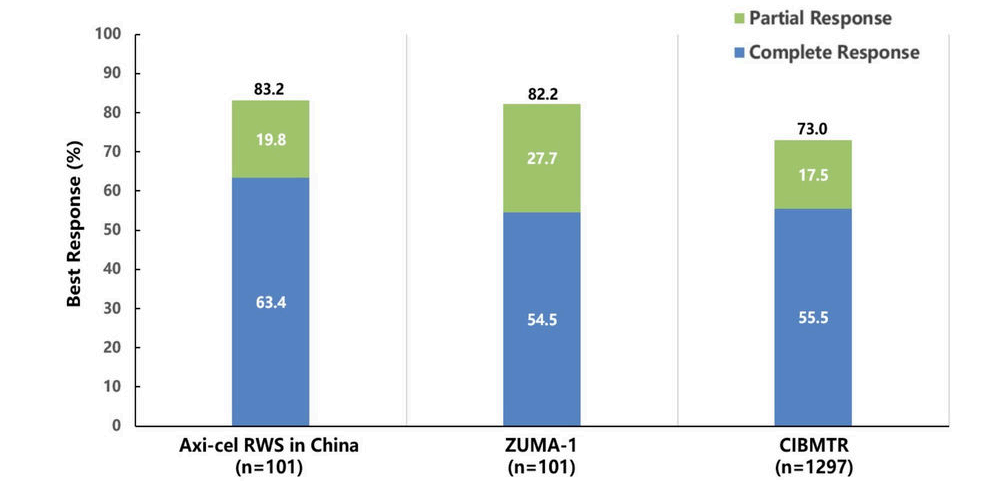
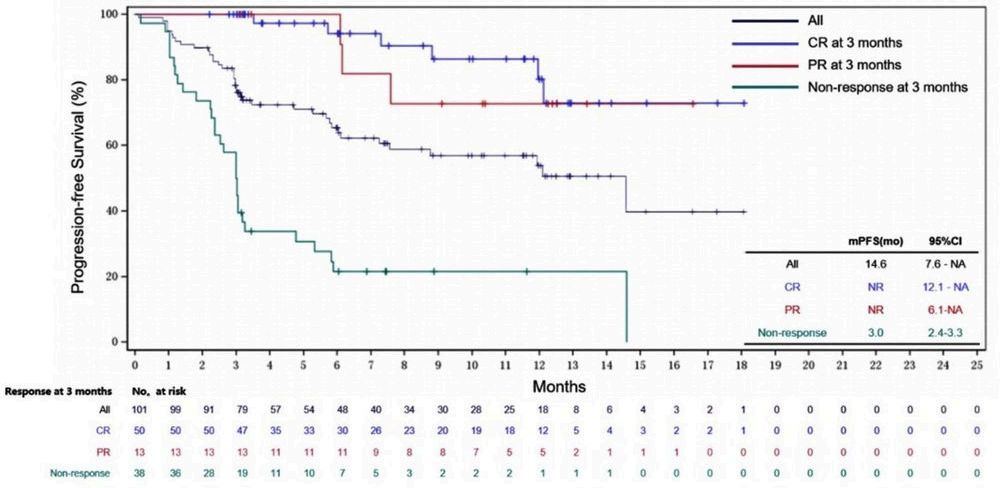
Interim analysis of the real-world study on Axi-cel treatment for R/R NHL in China
CAR-T Therapy Status—Established in Third Line, Challenging Second Line
As CAR-T therapy is increasingly applied in clinical settings, many factors affecting its efficacy have been identified, one of which is the line of treatment. Real-world data presented at the 2022 European Hematology Association (EHA) meeting showed that as the line of treatment increases, the complete response (CR) rate for CAR-T treatment in relapsed/refractory (R/R) DLBCL decreases, and survival worsens. At the 2023 American Society of Hematology (ASH) meeting, a multi-center retrospective propensity score-matched analysis from Iran, including 44 LBCL patients treated with Axi-cel from January 2019 to June 2023, with 22 patients each in second-line and third-line treatment, showed that Axi-cel in the second-line setting had a better objective response rate (ORR) and progression-free survival (PFS), with a similar rate of adverse events (AEs). The higher CAR-T expansion in the second-line treatment suggests that CAR-T products prepared earlier in treatment have better quality and a more favorable T-cell subtype.
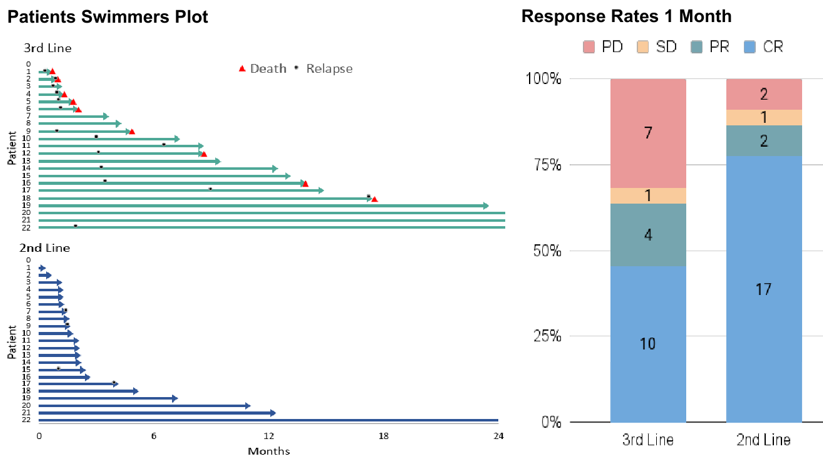

Iranian multicenter retrospective propensity score-matched analysis
Based on these findings and the exceptional performance of CAR-T therapy in DLBCL, several phase III CAR-T clinical trials have begun to challenge the second-line standard of care (SOC), but consistent results have yet to be achieved. The ZUMA-7 and TRANSFORM studies showed that compared to SOC, Axi-cel significantly improved event-free survival (EFS) [HR (95% CI) of 0.4 (0.31-0.51) and 0.35 (0.23-0.53), respectively]; however, the Belinda study showed no significant impact of Axi-cel on EFS compared to SOC [HR (95% CI) of 1.07 (0.82-1.4)], and this study has not yet reached its primary endpoint.
With the advent of the era of cellular immunotherapy, the role of transplantation in R/R DLBCL has been further refined. The 2022 version of the National Comprehensive Cancer Network (NCCN) guidelines suggests Axi-cel with bridging therapy for patients ineligible for transplantation but interested in CAR-T therapy, with related studies exploring this option. The PILOT study, an open-label, single-arm, multicenter phase II study, aims to evaluate the efficacy and safety of Liso-cel as a second-line therapy for R/R LBCL patients not planned for hematopoietic stem cell transplantation. The final analysis results of the PILOT study, announced at the 2023 ASH meeting after a median follow-up of 18.2 months, were consistent with the primary analysis, showing that in R/R LBCL patients not planned for transplantation, Liso-cel treatment resulted in a higher CR rate, longer DOR, and durable CR; with a median overall response duration of 23.3 months, and median duration for bCR patients not reached. Similarly, the ALYCANTE study assessed the efficacy of Axi-cel as a second-line treatment for R/R DLBCL patients ineligible for transplantation. Results presented at the 2023 EHA showed that 3 months post Axi-cel second-line treatment, patients achieved a complete molecular response (CMR) of 71%, compared to an estimated 12% CMR for SOC in previous studies; ORR and CR rates were 90.3% and 79% respectively; with a median follow-up of 12 months, median PFS was 11.8 months, median OS was not reached, and the 12-month OS rate was 78.3%; also, safety was manageable, with efficacy and safety consistent in patients aged ≥70 years.
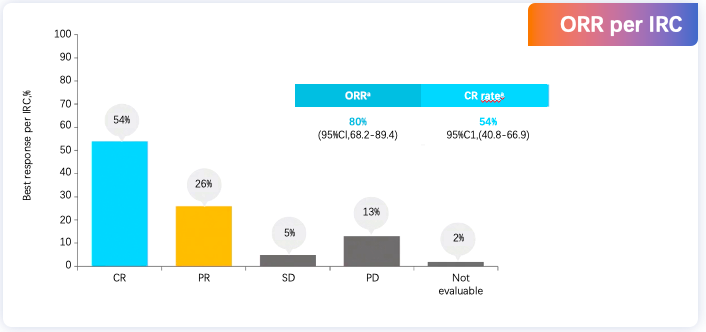

Final analysis of the PILOT phase II study
CAR-T as a Second-line Treatment—Several Pressing Issues Need Resolution
As a second-line treatment for DLBCL, CAR-T therapy currently faces several pressing issues that need to be addressed: (1) The role of bridging therapy: Bridging therapy is quite common in the real world, with 45%-53% of patients in the US and 78% of patients in Germany undergoing this treatment. The TRANSFORM study also reported a similar rate of bridging therapy (63%). Clinicians may still choose patients for bridging therapy based on the severity of the disease (especially volume and growth dynamics). (2) Optimal treatment for chemotherapy-sensitive patients: Autologous stem cell transplantation (ASCT) can cure about 50% of chemotherapy-sensitive patients, and up to 40% of patients are treated with ASCT while in partial remission (PR). CAR-T can also be used as a third-line treatment after ASCT relapse, with about 40% of patients achieving durable remission. It remains unclear how many patients are suitable for ASCT or can be cured through ASCT after failing CAR-T treatment if they first receive CAR-T therapy without undergoing ASCT. Moreover, patients relapsing after more than 12 months are more likely to be chemotherapy-sensitive, and it’s still uncertain whether second-line CAR-T therapy or salvage chemotherapy combined with ASCT is preferable for this group. (3) The significant cost issue of CAR-T therapy.
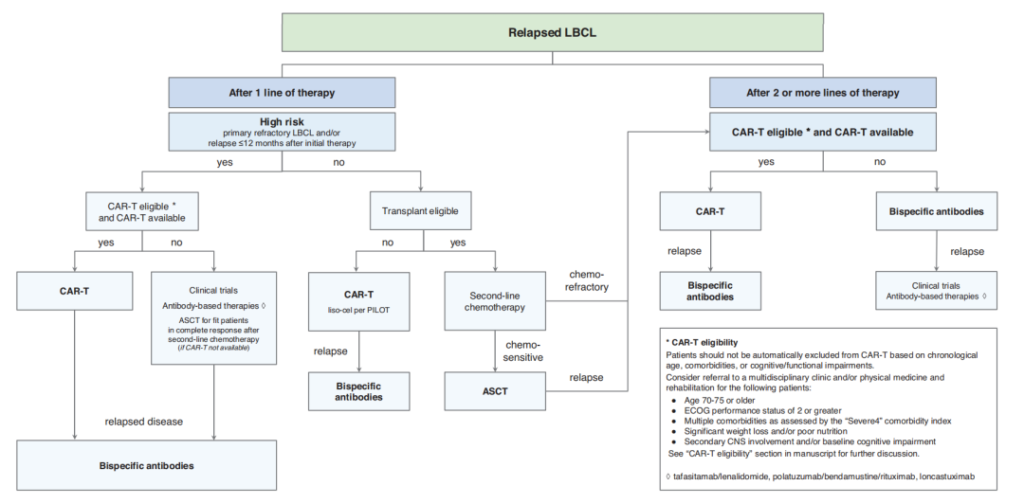
Treatment Flowchart for R/R DLBCL
Despite the FDA approval of anti-CD19 CAR-T cell therapy as a second-line treatment for high-risk LBCL (treatment of primary refractory disease or relapse within 12 months after first-line treatment), there are still several issues to consider regarding the actual implementation of this treatment, such as suitable histological subtypes; limitations related to age, comorbidities, and tumor biology; managing highly aggressive tumor behavior; whether to use holding treatments and bridging or salvage therapy before second-line CAR-T treatment; and how to proceed with bridging therapy, including whether to continue with the planned second-line CAR-T treatment if the response to bridging therapy is good.
CAR-T Optimization Strategies—Target Innovation, Enhanced Functionality
Target innovation, enhanced functionality, reduced side effects, and allogeneic cell therapies are the current four major trends in CAR-T therapy innovation. Single-target CAR-T cells often encounter antigen escape and clonality-negative relapse, while dual-target CAR-T cells aim to overcome these drawbacks by targeting tumor cells more accurately, showing better clinical efficacy and safety in various hematologic malignancies and even solid tumors. The novel anti-CD20/CD19 dual-target CAR-T (C-CAR039) in long-term follow-up results of patients with R/R B-cell NHL showed, with a median follow-up of 23.9 months, that C-CAR039 demonstrated good safety characteristics and deep, durable remissions in R/R B-NHL patients, especially in those with LBCL.
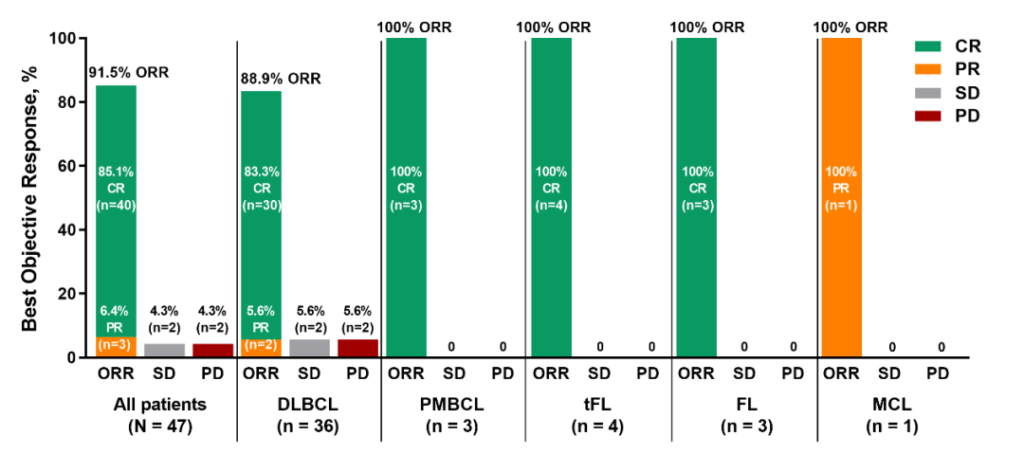
Long-term Follow-up Results of Novel Anti-CD20/CD19 Dual-Target CAR-T (C-CAR039) in R/R B-cell NHL Patients
In enhancing the functionality of CAR-T cells, Chinese scientists have made significant contributions, including optimizing the CAR structure, blocking immune checkpoint receptors, improving CAR-T cells through genetic modification, studying combination therapies, optimizing ex vivo culture protocols, and developing CAR-T cocktails. The first human trial results of the fourth-generation 4-1BB CAR-T product, HuCART19-IL18, for treating R/R lymphoma were presented at the International Conference on Malignant Lymphoma (ICML) in 2023. The data showed that HuCART19-IL18 treatment could achieve durable remission in patients with R/R CD19+ NHL who had previously received second-generation anti-CD19 CAR-T therapy. Additionally, multiple studies have shown that ASCT combined with CAR-T therapy is safe and effective for treating B-NHL, especially in R/R DLBCL patients.
Lymphoma treatment has entered the era of cellular immunotherapy, with several anti-CD19 CAR-T therapies approved for R/R LBCL patients. Key clinical trials and real-world studies (RWS) have demonstrated the significant efficacy of anti-CD19 CAR-T in R/R LBCL. To further improve the therapeutic status and efficacy of CAR-T, it is necessary to continue exploring more CAR-T treatment targets, develop new generations of CAR and multi-target CAR-T cells, and combine CAR-T with other therapies. For high-risk LBCL patients, the timing of CAR-T treatment is also a direction for future exploration.

Professor DeHui Zou
Chief Physician, Master’s degree holder, Master’s student supervisor
Assistant Director of the Lymphoma Diagnosis and Treatment Center
Chinese Academy of Medical Sciences & Peking Union Medical College Hospital (Institute of Hematology)
Standing Committee Member of the Lymphoma Committee of the Chinese Anti-Cancer Association
Chairman of the Lymphoma Committee of the Tianjin Anti-Cancer Association
Vice Leader of the Autologous Stem Cell Transplantation Working Group of CSCO
Vice Leader of the Lymphoma Study Group of the Hematologic Oncology Committee of the Chinese Anti-Cancer Association
Standing Committee Member of the Hematology Branch of the Chinese Geriatrics Society
Corresponding Editor of the Chinese Journal of Hematology


