
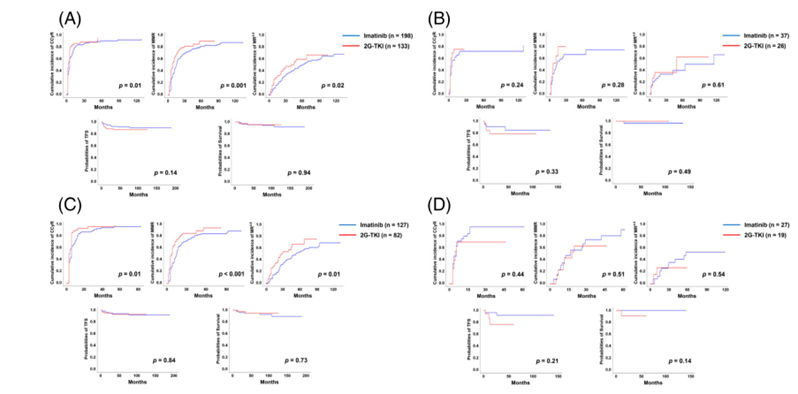
In July 2023, a study led by Professor Qian Jiang from Peking University Institute of Hematology was published in the international academic journal ——American Journal of Hematology (IF=12.8). The title of the study is “Imatinib compared with second-generation tyrosine kinase inhibitors in persons with chronic myeloid leukemia presenting in accelerated phase“. This study provides valuable evidence supporting the use of 2G-TKIs over imatinib as initial therapy for newly-diagnosed CML in the accelerated phase due to better cytogenetic and molecular response rates.
Chronic Myeloid Leukemia (CML) is a type of cancer that affects the blood cells and bone marrow and progresses through different phases: chronic, accelerated, and blast phase. The introduction of tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of CML, significantly improving patient outcomes. Imatinib, the first-generation TKI, has been a cornerstone of therapy, while second-generation TKIs (2G-TKIs) such as dasatinib, nilotinib, and others, have offered alternatives with different efficacy and safety profiles. The decision regarding which TKI to use as initial therapy is critical, especially for patients diagnosed in the more advanced accelerated phase of CML, where the risk of progression to blast phase and the associated poor prognosis are of particular concern. This setting demands a nuanced understanding of the therapeutic options to optimize patient outcomes. In light of this, a recent comprehensive study aimed to compare the responses and outcomes of imatinib and 2G-TKIs as initial therapy in newly diagnosed patients with CML in the accelerated phase. This comparison, derived from a large cohort across multiple centers in China, provides invaluable insights into the effectiveness and safety of these therapies, guiding clinicians in making informed decisions tailored to the unique needs of their patients.
The study’s findings that 2G-TKIs may offer superior rates of cytogenetic and molecular responses compared to imatinib in this patient population are clinically significant. Achieving complete cytogenetic response (CCyR), major molecular response (MMR), and deeper molecular responses like MR4.5 are critical milestones in the treatment of CML, associated with improved long-term outcomes, including survival. However, the finding that imatinib treatment was associated with worse transformation-free survival (TFS) raises concerns about the risk of progression to blast phase, underscoring the importance of choosing the right therapy from the onset of treatment.
These results have direct implications for clinical practice, suggesting that clinicians should consider starting treatment with a 2G-TKI in patients newly diagnosed with CML in the accelerated phase, especially those at higher risk of disease progression. However, the higher incidence of grade 3/4 hematologic adverse events (AEs) in the 2G-TKI cohort highlights the need for careful patient monitoring and may necessitate dose adjustments or temporary discontinuation of therapy to manage these AEs.
Comparing these findings to existing literature, previous studies have demonstrated the efficacy of 2G-TKIs in the chronic phase of CML, but data on their use in the accelerated phase have been limited. This study fills an important gap in the literature by providing evidence from a large, multicenter cohort specifically examining outcomes in the accelerated phase. The study’s use of propensity score matching to adjust for baseline differences between the cohorts adds to the robustness of the findings, although randomized controlled trials would be needed for definitive conclusions.
(Am J Hematol. 2023 Jul;98(7):E183-E186.)
The study provides valuable evidence supporting the use of 2G-TKIs over imatinib as initial therapy for newly-diagnosed CML in the accelerated phase due to better cytogenetic and molecular response rates. However, the associated higher risk of AEs and the observational nature of the study call for cautious interpretation of the results and further investigation in randomized controlled trials to establish the optimal treatment approach in this patient population.


