The approval of the long-acting injectable cabotegravir-rilpivirine in January 2021 marked a significant milestone in the medical realm. This dual-drug regimen has revolutionized the approach to HIV treatment by providing an effective and convenient alternative to daily oral medications. However, the question of how to best optimize access to this therapy in our clinics was an issue that required immediate attention. Consequently, a hypothesis was developed suggesting that cabotegravir-rilpivirine could be safely and effectively administered in a person’s own home, paving the way for an innovative study to test this theory.
Concept and Design of the Study
The study was designed as an Implementation Science Trial, which involved a meticulously planned 12-month intervention for individuals receiving cabotegravir-rilpivirine under the direction of their primary HIV provider. The safety of the participants was the top priority in this study, and thus, the first injection was always administered in the clinic to monitor the initial response of the patient. For subsequent injections, participants were given the autonomy to decide the location – in-clinic or at-home, providing them with an unprecedented level of control over their treatment. This level of flexibility in the treatment plan was believed to potentially increase adherence to the regimen, thus improving outcomes.
The financial aspect of the injection administration was covered by the study, alleviating any monetary concerns for the participants. However, participants were required to obtain the medication through insurance. For at-home injections, participants received and stored the medication in their refrigerators prior to a Licensed Practical Nurse (LPN) visit for administration. An additional safety measure was the provision for at-home participants to opt to have an epinephrine pen available in case of any adverse reactions.
The Administration Process
The study design required a complex coordination process that involved multiple key stakeholders: the patient, the provider, pharmacists, the study coordinator, and the LPN. This collaborative effort was essential for both in-clinic and at-home treatment. For at-home injections, a target treatment day was established in accordance with the LPN and participant’s schedule. The study coordinator then communicated the treatment day to the pharmacy to time the shipment date.
On the day of treatment, the participant removed the product from refrigeration while the LPN was in route for the visit. The LPN stayed for 15 minutes post-injection to ensure there were no post-injection reactions, thus ensuring the safety and wellbeing of the patient at all times. This intricate process was designed to ensure a seamless transition from in-clinic to at-home treatment administration.
Study Cohort and Research Findings
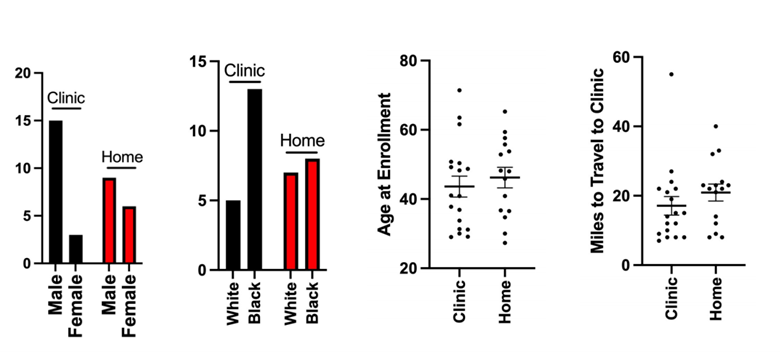
The study enrolled a diverse range of participants over a span of more than a year. Of these, a majority completed the study, with a small number remaining at the time of the report. Participants were divided into two groups based on their preference for the location of treatment administration – some opting for home treatment, while others chose the clinic.
One of the significant findings was that there were no significant differences between the cohorts in any parameters. This included the efficiency of medication administration or the rate of virologic suppression. This indicated that the location of administration, whether at home or in the clinic, did not significantly impact the effectiveness of the treatment or the adherence to the treatment schedule.
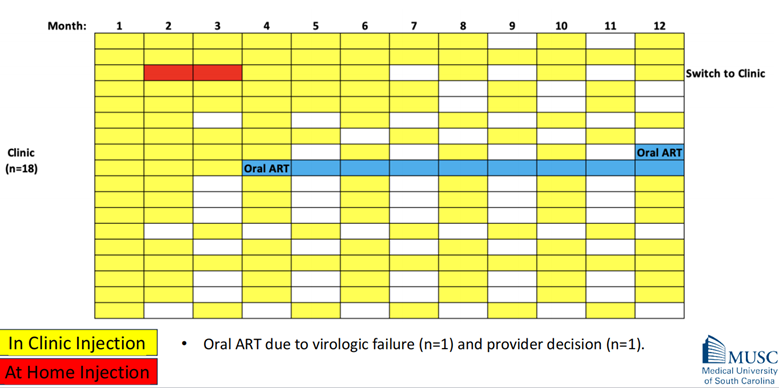
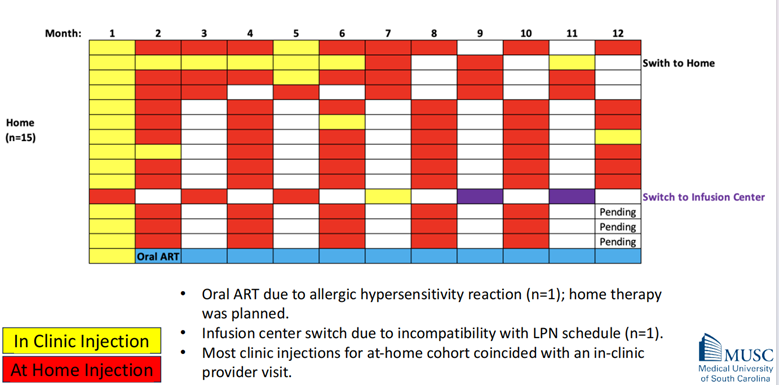
The study observed infrequent crossover between the two cohorts. This means that once a participant had chosen a preferred location (home or clinic) for their injections, they rarely switched to the other option. A small number of participants in the clinic cohort switched to at-home treatment, and similarly, a few in the home cohort had to revert to oral antiretroviral therapy (ART) due to various reasons.
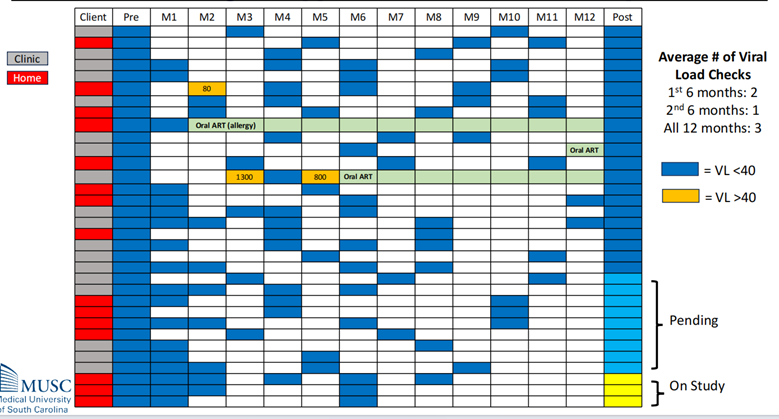
Virologic Suppression and Participant Satisfaction
Virologic suppression, a crucial indicator of the effectiveness of HIV treatment, was sustained in the majority of participants. Only a few participants had to revert to oral ART. This showcases the overall effectiveness of the cabotegravir-rilpivirine regimen, regardless of the administration location.
An important aspect of treatment success is patient satisfaction, and this study demonstrated high satisfaction levels among participants. The participants were asked to rate their satisfaction with their current treatment on a scale of 0-6, with 6 being very satisfied. The mean satisfaction score was 5.9, indicating high contentment with both in-clinic and at-home treatment options.
Extent of Crossover and Adverse Events
The extent of crossover between the cohorts was infrequent. This means that once a participant had chosen a preferred location (home or clinic) for their injections, they rarely switched to the other option. This could indicate a high level of satisfaction with their chosen method of administration.
In terms of adverse events, injection site pain/soreness was common but not severe. This was a minor side effect and did not disrupt the treatment process.
Post-Intervention Participant Interviews
Post-intervention participant interviews were conducted to gain deeper insights into the participants’ experiences and perceptions. The interviews were audio-recorded, transcribed, and coded by independent coders. The qualitative analysis process identified five overarching themes: general impressions, strengths of injectable medication, negatives of injectable medication, treatment setting preferences, and reasons for preferring home setting.
When it came to treatment setting preferences, most participants who selected at-home treatment expressed frustration in not being able to continue at-home treatment after finishing the study. The reasons for preferring at-home treatment were varied and included factors such as distance/travel, time, safety of home, and convenience.
The study concluded that home administration of injectable cabotegravir-rilpivirine is safe, effective, and associated with high satisfaction. The findings support exploring efforts to make injectable ART more accessible in non-clinical settings.
However, the study also acknowledged that this innovative project required extensive time and coordination efforts from clinical team members, which may be difficult to reproduce, sustain, and implement in busy clinical practice. Future research and implementation strategies need to address these challenges to make this innovative treatment approach more widely available to HIV patients.


