
Editor’s note: At the 2025 European Society for Medical Oncology (ESMO) Annual Congress, Chinese innovative drugs and clinical research drew global attention. During the meeting, Xichun Hu, Professor at Fudan University Shanghai Cancer Center, presented a Late-Breaking Abstract (LBA) detailing the pivotal phase III study in which China’s first original HER2-targeted antibody–drug conjugate (ADC), A166 (budotituzumab vedotin), successfully challenged T-DM1 in the second-line setting for HER2-positive breast cancer. Based on this study, A166 received approval from the National Medical Products Administration (NMPA) on October 17, 2025, bringing a new standard-of-care option to clinical practice. Meanwhile, Man Li, Professor at the Second Hospital of Dalian Medical University, also presented an LBA reporting results from OptiTROP-Breast02, evaluating China’s first original Trop-2 ADC, sac-TMT (SKB264), in HR+/HER2- advanced breast cancer, demonstrating prolonged progression-free survival (PFS).
At this global gathering, under the moderation of Wenjin Yin (Renji Hospital, Shanghai Jiao Tong University School of Medicine), Hope S. Rugo from the City of Hope Comprehensive Cancer Center joined Professors Xichun Hu and Man Li for a high-level discussion on breakthrough achievements and the global prospects of China’s innovative ADCs.
A New Rising Star in HER2-Targeted ADCs: A166 Brings New Hope to Second-Line Treatment
Wenjin Yin:
At this year’s ESMO Congress, Prof. Xichun Hu presented the results of the head-to-head study comparing China’s HER2 ADC A166 with T-DM1 for HER2-positive advanced breast cancer. Could you summarize the main findings? How does A166 differ from other HER2-targeted ADCs in terms of design and clinical performance?
Xichun Hu:
We presented the results of this national, multicenter, phase III randomized clinical trial comparing A166 with T-DM1 in patients with HER2-positive breast cancer previously treated with trastuzumab and taxanes. A total of 365 patients were enrolled across China, with roughly half previously receiving first-line therapy and half receiving two or more prior lines of HER2-targeted therapy.
Patients were randomized 1:1 to A166 or T-DM1. The primary endpoint was PFS assessed by an Independent Review Committee (BICR). During the study, the first interim analysis reported the initial PFS results; at the second analysis, the final PFS results were disclosed. If superiority in PFS was confirmed, overall survival (OS) testing would proceed.
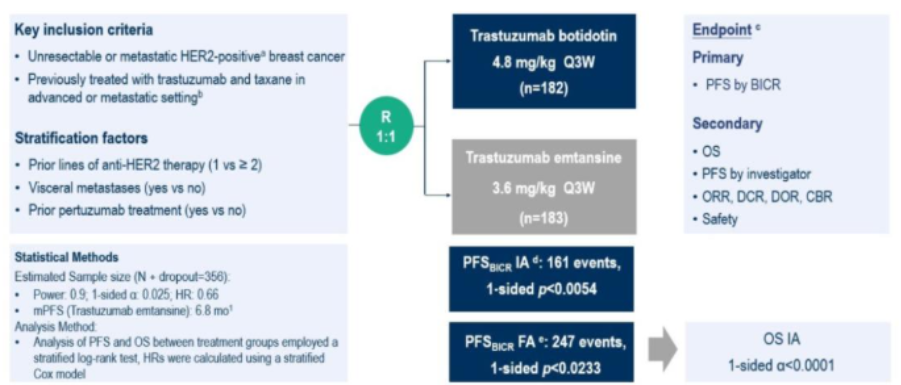
Phase III Study Design of A166
The newly presented results showed that in the T-DM1 arm, the median PFS (mPFS) was 4.4 months, while A166 extended mPFS to 11.1 months (HR 0.39; 95% CI: 0.30–0.51; P < 0.0001), meeting the criteria for a positive primary endpoint. Further confirmation of OS benefit is eagerly awaited. [1]

BICR-Assessed PFS in the Phase III A166 Study
Among the enrolled patients, approximately 45% had received prior pertuzumab and about 60% had received a prior TKI, reflecting real-world treatment patterns in China. The positive results presented will have substantial impact on clinical practice.
At this year’s ESMO, subgroup analyses from DESTINY-Breast05, DESTINY-Breast11, and DESTINY-Breast09 were released, collectively indicating that trastuzumab deruxtecan (T-DXd) is rapidly moving into earlier treatment lines. However, for patients who progress after T-DXd, no effective treatment options are currently available. The emergence of A166 is timely, providing a new therapeutic option for this post-T-DXd population.
In addition, ILD/pneumonitis, a major clinical concern with T-DXd, limits its use in patients with a history of such events. A166 offers a viable alternative for this subgroup.
Hope S. Rugo:
MMAF-based cytotoxic payloads are associated with ocular toxicity. ADCs such as A166 and ARX788, which use MMAF, share this issue. Since you have been deeply involved in clinical studies of both agents, how do you recommend managing ocular toxicity?
Xichun Hu:
Ocular toxicity from these agents is non–receptor-dependent; the payload can be taken up directly by ocular tissues, leading to adverse events. In clinical studies, we use artificial tears and deproteinized calf serum eye drops to prevent or alleviate ocular symptoms.
Hope S. Rugo:
In our practice, we collaborate closely with ophthalmologists and use prophylactic eye drops to reduce excessive absorption of the cytotoxic payload. This approach helps maintain efficacy while improving patients’ quality of life. Since A166 and similar agents are highly effective, supportive multidisciplinary care helps patients not only live longer but also maintain good daily functioning.
Wenjin Yin:
From your perspective, what unmet needs remain in HER2-positive advanced breast cancer? How will the availability of A166 reshape the treatment landscape in China?
Xichun Hu:
In this study, A166 achieved a clearly positive PFS result, and we are optimistic about its eventual overall survival benefit. If OS improvement is confirmed, A166 will undoubtedly become a new standard of care.
With the rapid advancement of ADCs—T-DM1, T-DXd, and now A166—patients whose disease progresses on first-line HER2-targeted therapy will have new therapeutic choices. In registration studies, the objective response rate (ORR) of T-DXd in DESTINY-Breast03 was 79.7%, while A166’s phase III study demonstrated a similarly high ORR of 76.9%, confirming its strong antitumor activity.
A166 also shows a distinctive safety profile, offering a safer second-line option. Based on both efficacy and safety, A166 addresses two key clinical needs:
- Overcoming resistance following progression on HER2-targeted therapy
- Providing a safer alternative for patients unsuitable for T-DXd due to toxicity concerns
Together, these advantages position A166 to reshape the second-line treatment landscape for HER2-positive advanced breast cancer in China.
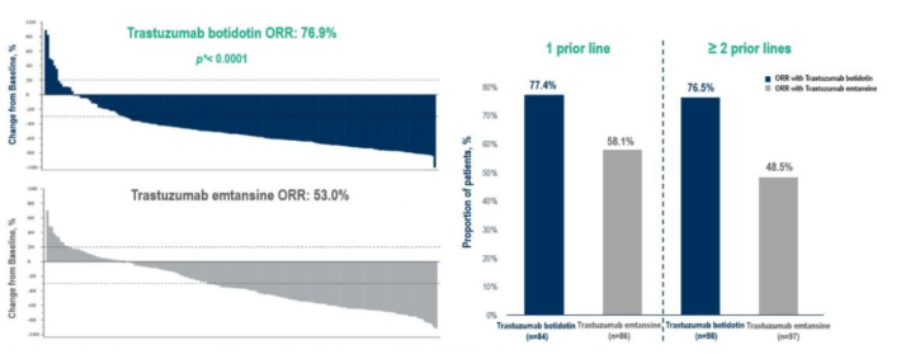
BICR-Assessed Response Outcomes in the Phase III A166 Study
OptiTROP-Breast02 Breaks New Ground — sac-TMT Advances Again
Wenjin Yin:
In recent years, Trop-2–targeted ADCs have made remarkable progress in HR+/HER2- breast cancer. At this ESMO Congress, Prof. Man Li presented the phase III OptiTROP-Breast02 study—the first registration study of a China-developed Trop-2 ADC in an all-Chinese HR+/HER2- population. How should we interpret the significance of sac-TMT’s achievements in breast cancer?
Man Li:
As the first Trop-2 ADC developed in China, sac-TMT has drawn significant attention since its launch. Previously, based on the strong efficacy demonstrated in OptiTROP-Breast01 for second-line treatment of advanced triple-negative breast cancer (TNBC)[6], both the 2025 CSCO Breast Cancer Guidelines[7] and the 2025 CACA & CMA Breast Cancer Guidelines (v2.0 Essential Edition)[8] recommended sac-TMT as a standard therapeutic option.
At this year’s ESMO Congress, the OptiTROP-Breast02 study results were presented, focusing on HR+/HER2- advanced breast cancer. Eligible patients had received 1–4 prior lines of chemotherapy, and all had previously been treated with CDK4/6 inhibitors, endocrine therapy, and taxanes. A total of 399 patients were enrolled—each previously exposed to CDK4/6i and at least one prior chemotherapy regimen. Patients were randomized 1:1 to receive sac-TMT or investigator’s-choice chemotherapy (ICC).

Study Design of OptiTROP-Breast02
The results demonstrated that sac-TMT significantly prolonged median PFS compared with ICC: 8.3 months vs. 4.1 months (HR 0.35; 95% CI: 0.26–0.48; P < 0.0001). Although overall survival (OS) remains immature, sac-TMT reduced the risk of death by 63% compared with ICC (HR 0.33; 95% CI: 0.18–0.61).
The objective response rate (ORR) was also higher in the sac-TMT arm at 41.5%, versus 24.1% in the ICC arm, indicating superior tumor response.
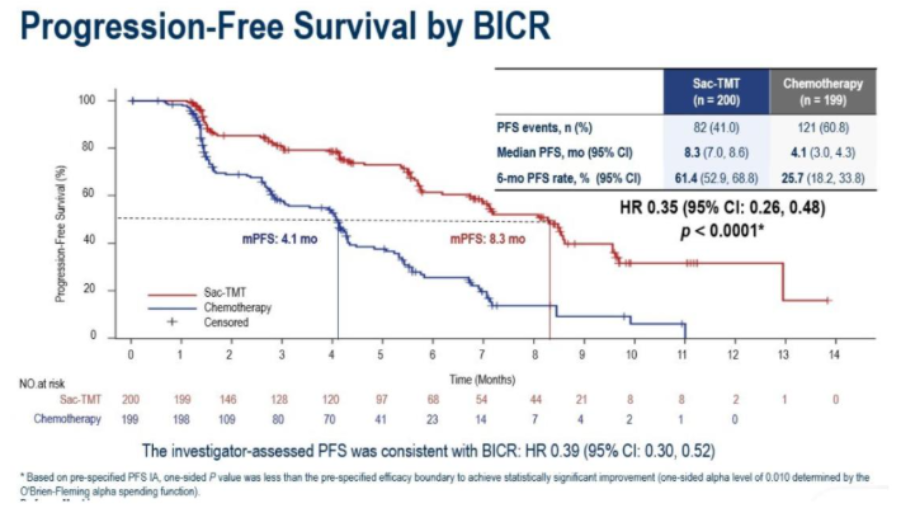
BICR-Assessed PFS in the OptiTROP-Breast02 Study
In terms of safety, sac-TMT demonstrated a profile consistent with previous studies, with no new safety signals observed. The incidence of grade ≥3 treatment-related adverse events (TRAEs) was comparable between groups: 62.0% in the sac-TMT arm versus 64.8% in the ICC arm. Hematologic toxicities were the most common TRAEs in both groups.
In the sac-TMT arm, the most frequent non-hematologic TRAE was oral mucositis, predominantly grade 1–2. The incidence of ILD/pneumonitis was low (1.5% with sac-TMT vs. 1.0% with ICC, all grade 1–2). No TRAEs led to treatment discontinuation, and no treatment-related deaths were reported during the study.
This predictable and manageable safety profile supports smooth treatment delivery and helps maintain long-term quality of life for patients.[9]
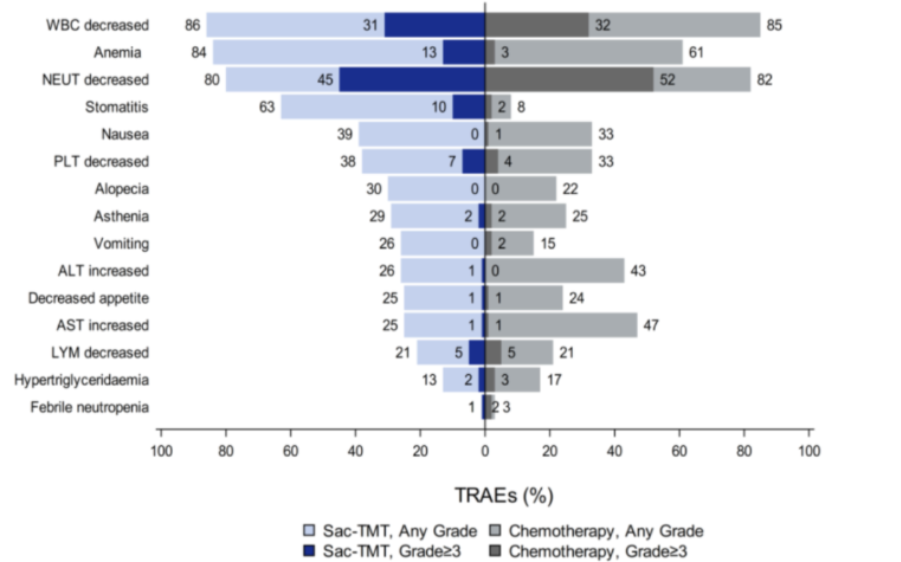
Safety Profile of OptiTROP-Breast02
Overall, the OptiTROP-Breast02 study demonstrated both superior efficacy and reliable safety. The performance of sac-TMT in HR+/HER2- advanced breast cancer was fully comparable to that of international Trop-2 ADCs, underscoring the robustness of Chinese innovative drug development.
At this ESMO Congress, OptiTROP-Breast02 was presented as a Late-Breaking Abstract and received broad recognition from international experts, once again confirming that the quality of China’s original Trop-2 ADCs is now aligned with leading global standards. Looking ahead, under the leadership of Prof. Hope S. Rugo and others, we hope to further advance global clinical studies of sac-TMT so that this Chinese-developed drug can benefit breast cancer patients worldwide.
Wenjin Yin:
At present, three Trop-2 ADCs have been approved and are in clinical use in China. Prof. Rugo, you have extensive experience with all three in clinical trials. What are your views on these agents and your expectations for their role in cancer treatment?
Hope S. Rugo:
The OptiTROP-Breast02 study has produced very impressive results, including higher response rates and longer PFS. It is important to note that we currently lack head-to-head comparison data among the three Trop-2 ADCs. As emphasized at this ESMO meeting, the choice of ADC in clinical practice will still largely depend on patient characteristics and safety considerations for the foreseeable future.
What is clear is that sac-TMT is a highly effective treatment for HR+/HER2- breast cancer and has also shown very good activity in TNBC. We are now conducting the international TroFuse study series, with designs that closely mirror the Chinese trials, and have seen encouraging early data. The main adverse events associated with sac-TMT are hematologic toxicity and stomatitis, with relatively mild nausea and no ILD observed to date. In TroFuse, sac-TMT has shown good tolerability while maintaining efficacy.
We cannot yet say which ADC will ultimately be the preferred option, but ongoing research across different patient populations will help clarify their optimal use and guide clinical practice in the future.
Technological Innovation and International Collaboration: Chinese Innovation Goes Global
Wenjin Yin:
Including at this year’s ESMO, Chinese innovative drugs are increasingly presenting strong data on the international stage. What suggestions do you have for helping Chinese innovative drugs move onto the global arena?
Xichun Hu:
A166 is one of the representative examples of Chinese innovative drugs going global and incorporates several patented technologies. It uses a site-specific conjugation strategy to ensure structural homogeneity, with a drug–antibody ratio (DAR) of 2:1. A Val-Cit linker is used, which remains stable in the peripheral circulation but is specifically cleaved by cathepsin B, an enzyme highly expressed in tumor cells, to release the cytotoxic payload intracellularly.
Drug innovation fundamentally relies on technological innovation. Based on the structural design and mechanism of A166, the phase I trial demonstrated an ORR of 73.9% and a median PFS of 12.3 months, giving us strong confidence to proceed with further clinical development.[10] Therefore, in the phase III design, we chose a head-to-head comparison with T-DM1—the then standard second-line treatment for HER2-positive advanced breast cancer. The positive results presented at ESMO validate the soundness of this strategy.
Regarding ocular toxicity, which many clinicians are concerned about, our clinical experience suggests that A166 is generally well tolerated. Only 2 patients (1.1%) discontinued treatment due to ocular events. We recommend intensified ophthalmologic monitoring during the first three months of A166 therapy, together with preventive measures; any emerging symptoms can then be managed promptly based on ophthalmology input.
In summary, advances in China’s biopharmaceutical and biotechnology sectors have strongly propelled ADC development, and this has been confirmed in clinical trials. Technological innovation is the cornerstone driving the growth of Chinese new drugs.
Man Li:
Chinese innovative drugs have already gained international recognition, but their global journey has only just begun. Looking ahead, Chinese pharmaceutical companies need to further strengthen original innovation at the source—exploring new targets, new technologies, and new modalities. By conducting global multicenter clinical trials, by collaborating with world-leading experts such as Prof. Xichun Hu and Prof. Hope S. Rugo, and by leveraging strategic partnerships or mergers and acquisitions, we can accelerate the global launch of Chinese innovative drugs and benefit cancer patients both in China and worldwide.
Hope S. Rugo:
China has invested tremendous human and material resources into new drug development and has made rapid progress. In my view, international collaboration is the best way to help Chinese innovative drugs enter the global market. For example, sac-TMT’s partnership between Kelun Biotech and Merck has significantly accelerated its international development. Other modalities, such as bispecific antibodies, are also flourishing under similar models.
These collaborations not only speed up clinical adoption but also allow us to deeply investigate pharmacogenomic profiles, prior treatment exposures, and population differences across international patient cohorts—greatly enhancing our understanding of ethnic and biological variability. For me, the progress of innovative drugs under international collaboration and the continued publication of high-quality research results are truly exciting.
References
[1] Xichun Hu, Jian Zhang, Quchang Ouyang, et al. LBA24 – Trastuzumab botidotin vs trastuzumab emtansine (T-DM1) in HER2-positive unresectable or metastatic breast cancer: Results from a randomized phase III study. 2025 ESMO LBA24.
[2] Charles E. Geyer, Yeon Hee Park, Zhi-Ming Shao, et al. LBA1 – Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with high-risk human epidermal growth factor receptor 2–positive (HER2+) primary breast cancer (BC) with residual invasive disease after neoadjuvant therapy (tx): Interim analysis of DESTINY-Breast05. 2025 ESMO LBA1.
[3] Nadia Harbeck, Shanu Modi, Lajos Pusztai, et al. 291O – DESTINY-Breast11: Neoadjuvant trastuzumab deruxtecan alone (T-DXd) or followed by paclitaxel + trastuzumab + pertuzumab (T-DXd-THP) vs SOC for high-risk HER2+ early breast cancer (eBC). 2025 ESMO 291O.
[4] Sibylle Loibl, Zefei Jiang, Romualdo Barroso-Sousa, et al. LBA18 – Trastuzumab deruxtecan (T-DXd) + pertuzumab (P) vs taxane + trastuzumab + pertuzumab (THP) for patients (pts) with HER2+ advanced/metastatic breast cancer (a/mBC): Additional analyses of DESTINY-Breast09 in key subgroups of interest. 2025 ESMO LBA18.
[5] Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med. 2022;386(12):1143-1154. doi:10.1056/NEJMoa2115022
[6] Yin Y, Fan Y, Ouyang Q, et al. Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial. Nat Med. 2025;31(6):1969-1975. doi:10.1038/s41591-025-03630-w
[7] CSCO Breast Cancer Guidelines Committee. Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2025. People’s Medical Publishing House, Beijing, April 2025.
[8] Secretariat of the “Breast Cancer Diagnosis and Treatment Guidelines and Standards” Editorial Office, Chinese Anti-Cancer Association & Chinese Medical Association Oncology Branch. Breast Cancer Diagnosis and Treatment Guidelines and Standards (2025 Essential Edition, v2.0). Fudan University Press, Shanghai, June 2025.
[9] Man Li, Ying Fan, Huihui Li, et al. LBA23 – Sacituzumab tirumotecan (sac-TMT) vs investigator’s choice of chemotherapy (ICC) in previously treated locally advanced or metastatic hormone receptor-positive, HER2-negative (HR+/HER2-) breast cancer (BC): Results from the randomized, multi-center phase III OptiTROP-Breast02 study. 2025 ESMO LBA23.
[10] Zhang J, Liu R, Gao S, et al. Phase I study of A166, an antibody–drug conjugate in advanced HER2-expressing solid tumours. NPJ Breast Cancer. 2023;9(1):28. doi:10.1038/s41523-023-00522-5

Xichun Hu, MD, Professor
Fudan University Shanghai Cancer Center

Hope S. Rugo, MD, Professor
Helen Diller Family Comprehensive Cancer Center
University of California, San Francisco (UCSF)

Man Li, MD, Professor, Doctoral Supervisor

Wenjin Yin, MD, Professor
Renji Hospital, Shanghai Jiao Tong University School of Medicine

