TROP2 (Trophoblast Cell-Surface Antigen 2) is highly expressed in various solid tumors, making it a hot target in the field of oncology. The development of antibody-drug conjugates (ADCs) targeting this antigen has garnered significant attention. At the 2023 European Society for Medical Oncology (ESMO) Congress held in Madrid, Spain from October 20th to 24th, an innovative TROP2-ADC called SKB264 (MK-2870), jointly developed by CoromboTech and Merck, was presented in an oral report from a phase I/II basket trial for the treatment of advanced hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC) (Abstract number: 380MO). The study results confirm that SKB264 exhibits manageable safety and significant anti-tumor activity in HR+/HER2- metastatic breast cancer patients. Professor Yongmei Yin presented the research and its key findings at the conference.

Research Background
TROP2 is overexpressed in HR+/HER2- metastatic breast cancer and has been associated with poor patient outcomes. SKB264 is a cleverly designed ADC targeting TROP2, composed of a humanized anti-TROP2 monoclonal antibody, a stability-optimized CL2A linker (with antibody conjugation achieved via methylsulfonylpyrimidine for irreversible linkage), and the small molecule toxin T030 (a topoisomerase I inhibitor). The drug-to-antibody ratio (DAR) averages 7.4. This study marks the first public report of this innovative drug’s use in a phase II cohort of HR+/HER2- metastatic breast cancer patients.
Research Methods
HR+/HER2- (including HER2 low-expression and HER2-negative) metastatic breast cancer patients received SKB264 at a dose of 5 mg/kg every two weeks (Q2W) until disease progression or intolerable toxicity. Enrolled patients had previously failed endocrine therapy and received at least one prior chemotherapy. Tumor assessments were performed by researchers every 8 weeks based on RECIST v1.1 criteria.
Research Results
As of April 12, 2023, the study enrolled 41 patients with a median age of 50 years, with 61% of patients having an ECOG PS score of 1. The median follow-up time for the study was 8.2 months.
The primary adverse events were hematological toxicities, with the most common grade ≥3 treatment-related adverse events (TRAEs) being neutropenia (36.6%), leukopenia (22%), anemia (14.6%), and thrombocytopenia (9.8%). Most hematological toxicities occurred within the first two months of treatment and recovered with the use of G-CSF or erythropoiesis-stimulating agents without requiring blood transfusions. Dose reductions due to TRAEs occurred in 17.1% (7/41) of patients, with no treatment discontinuations or deaths due to TRAEs. Gastrointestinal TRAEs were rare, and no drug-related neuropathy or interstitial lung disease/pneumonitis was observed.
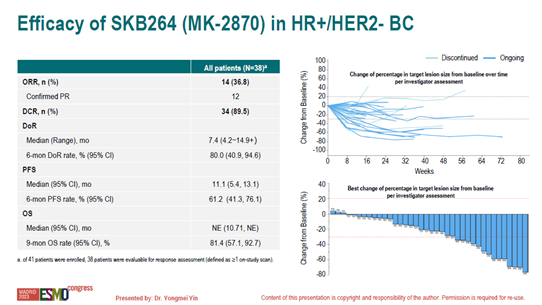
Among the 38 evaluable patients, 71% had low HER2 expression, 47% were primary endocrine therapy-resistant, and 79% had received ≥2 lines of chemotherapy for metastatic breast cancer, with all patients having prior exposure to taxane-based therapy, and 65.8% had received CDK4/6 inhibitors. The objective response rate (ORR) was 36.8%, disease control rate (DCR) was 89.5%, 6-month duration of response (DoR) was 80%, and median progression-free survival (PFS) was 11.1 months. All subgroups benefited, including HER2 low expression and negative, primary and secondary endocrine resistance, and prior use or non-use of CDK4/6 inhibitors.
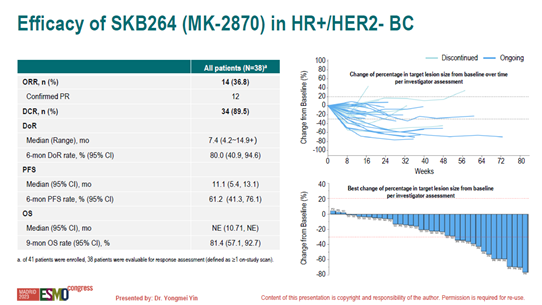
Research Conclusion
SKB264 demonstrates manageable safety and promising anti-tumor activity in HR+/HER2- metastatic breast cancer patients. Currently, two phase III clinical trials are being planned for HR+/HER2- metastatic breast cancer patients: one domestic study for patients who have received at least one line of chemotherapy in the metastatic setting and another global study for patients who have not received chemotherapy in the metastatic setting.
Summary
Previously, SKB264 has shown favorable efficacy and manageable safety in heavily pretreated patients with metastatic triple-negative breast cancer (TNBC) and non-small cell lung cancer (NSCLC). In this ESMO Congress, it has once again demonstrated substantial therapeutic potential in HR+/HER2- metastatic breast cancer patients, further boosting confidence in the development of more extensive studies in this field.
At the 2022 SABCS Annual Meeting, SKB264 released phase II expansion study data for locally advanced or metastatic TNBC patients. In 55 evaluable patients, the ORR was 43.6%, DCR was 80%, and patients with high TROP2 expression had an ORR of 55.2%. Median DoR was 11.5 months, median PFS was 5.7 months, median overall survival (OS) was 14.6 months, and the 12-month OS rate was 66.4%.
At the 2023 ASCO Annual Meeting, SKB264 presented phase II expansion study data for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who had failed EGFR-TKI therapy. In 39 evaluable patients, the ORR was 43.6%, DCR was 94.9%, median DoR was 9.3 months, 6-month DoR rate was 77%, and the 12-month OS rate was 70.6%. In the EGFR mutation subgroup, the ORR was 60%, DCR was 100%, median DoR was 9.3 months, median PFS was 11.1 months, and the 12-month OS rate was 80.7%.
The released study data suggests that SKB264 has significant potential in treating advanced triple-negative breast cancer, HR+/HER2- breast cancer, and EGFR-TKI-resistant NSCLC, making it one of the most promising ADCs in its class.
Based on the impressive research data in breast and lung cancer, SKB264 has been granted three Breakthrough Therapy designations by the China National Medical Products Administration (CDE): one for monotherapy in locally advanced or metastatic TNBC, another for EGFR-TKI-resistant locally advanced or metastatic EGFR-mutated NSCLC, and a third for locally advanced or metastatic hormone receptor (HR)-positive, HER2-negative breast cancer with at least second-line systemic therapy.
In the breast cancer field, a phase III registration study for SKB264 as monotherapy in patients with locally advanced or metastatic triple-negative breast cancer who have received at least second-line systemic treatment has achieved its primary endpoints and plans to submit a marketing application by the end of the year. A phase III registration study for SKB264 as monotherapy in patients with locally advanced or metastatic HR+/HER2- breast cancer who have failed at least first-line chemotherapy has been approved for initiation. A phase II study is ongoing for SKB264 in combination with KL-A167 (anti-PD-L1 monoclonal antibody) as first-line treatment for HER2-negative breast cancer. Additionally, multiple lung cancer registration studies are progressing rapidly, and combination therapies and applications in other solid tumors are actively being explored. Expect more research results to be released, bringing hope to cancer patients worldwide!

