Editor’s note:
The 2023 ESMO Annual Meeting took place in Madrid, Spain, from October 20th to 24th. As a prestigious annual event in the field of oncology, it unveils cutting-edge advancements in cancer research each year. This year, Chinese scholars made remarkable contributions, with many domestic experts presenting posters or giving oral presentations in various fields. Professor Feng Wang from the Department of Oncology at the First Affiliated Hospital of Zhengzhou University had two outstanding research findings included in this year’s conference. We had the privilege of interviewing Professor Feng Wang at the event, asking him to discuss the recent developments in esophageal and gastric cancer research in China.
Oncology Frontier: First, we’d like to thank you for agreeing to this interview. Could you share your main takeaways and experiences from attending this year’s ESMO conference?
Professor Feng Wang:
We’ve noticed that this year’s ESMO conference was quite crowded, and it was my first time attending an ESMO conference since the pandemic. I’m delighted to see more Chinese experts’ participation and contributions at this year’s ESMO.
With the development of innovative pharmaceutical companies in China, clinical scholars in our country are conducting numerous clinical trials, resulting in a constant stream of research achievements. Particularly at this year’s ESMO conference, we saw many domestic experts presenting posters or giving oral presentations in various fields, showcasing the outstanding contributions of Chinese clinical professionals.
In the future, I believe that we will see more research results in certain tumor types with Chinese characteristics, such as esophageal cancer, gastric cancer, liver cancer, and nasopharyngeal cancer, presented to the global community. In recent years, Chinese experts have been gradually accumulating rich experience in clinical trials. I hope that the voices of Chinese scholars will continue to strengthen at large international conferences such as ESMO and ASCO and play a leading role in various tumor types.
Oncology Frontier: Congratulations on having two of your research studies selected as abstracts for this year’s ESMO conference. One of them is about the latest updates on the use of Anlotinib in combination with PD-L1 inhibitors as a first-line treatment for advanced esophageal squamous cell carcinoma. Could you please discuss the main content and clinical significance of this study?
Professor Feng Wang:
My main research focus is on esophageal and gastric cancer. Esophageal cancer is the eighth most common cancer worldwide and the sixth leading cause of cancer-related deaths. In China, it ranks sixth in terms of incidence and fourth in terms of mortality. China can be considered a major country for esophageal cancer. Currently, the early diagnosis rate is relatively low, and most patients are in advanced stages when they seek medical attention.
In the past, the primary treatment for esophageal cancer, especially in advanced stages, was systemic chemotherapy. However, chemotherapy offered limited survival benefits and came with significant adverse effects, with more than 60-70% of patients experiencing Grade 3 or higher toxic side effects. Therefore, the benefit to patients was limited.
Since 2017, with the advent of the immunotherapy era, immunotherapy has shown superiority over single-agent chemotherapy in the second and third-line treatments for esophageal cancer, becoming the new standard treatment for esophageal cancer after progression. In recent years, the combination of immunotherapy and chemotherapy has yielded positive results in large randomized clinical trials for first-line treatment of esophageal cancer. However, it is clear that while the introduction of immunotherapy has significantly improved the survival of patients, the survival period for advanced esophageal cancer patients has not yet reached 20 months, leaving room for improvement in terms of efficacy. Additionally, the combination of chemotherapy and immunotherapy still leads to toxic side effects in over 60% of patients, especially those with Grade 3 or higher toxic side effects. Therefore, in the future, in the optimization of esophageal cancer treatment regimens, we aim to not only enhance the efficacy of existing treatments but also reduce the toxic side effects, thereby improving patient tolerance and quality of life.
One aspect of our exploration is to investigate more combination therapies, such as adding new anti-angiogenic drugs on top of chemotherapy and immunotherapy, forming a four-drug combination regimen. Currently, Merck is conducting the LEAP-014 study, which combines Anlotinib with chemotherapy and immunotherapy as a base. However, Chinese experts have observed, in previous small-sample Phase II clinical studies, that the four-drug combination regimen resulted in Grade 3 or higher toxic side effects as high as 80%. Although it significantly improved efficacy, patient tolerance was low. We understand that patients with advanced esophageal cancer have poor dietary and physical condition, low tolerance, and low treatment completion rates. Therefore, we hope to eliminate chemotherapy and instead combine anti-angiogenic drugs with immunotherapy to create a “targeted+immunotherapy” regimen. We want to see if this approach can improve patient outcomes while significantly reducing toxic side effects, thereby enhancing patient tolerance and treatment completion. Based on this, our ALTER-E003 study, conducted in collaboration with BeiGene, uses Anlotinib in combination with TQB2450 (PD-L1 monoclonal antibody) in a “chemo-free” approach in the first-line treatment of advanced esophageal cancer. Preliminary data has shown very favorable short-term efficacy and survival. The overall response rate (ORR) reported at ESMO is 69.6%, which I believe is quite comparable to the efficacy of chemotherapy combined with immunotherapy.
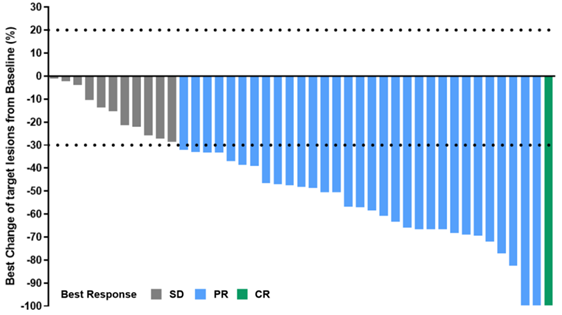
“Figure 1 Disease Response Rate in 43 Patients Treated with Anlotinib in Combination with TQB2450”
Additionally, another major study endpoint, progression-free survival (PFS), has reached 9.92 months, significantly better than the 6-7 months of PFS seen with chemotherapy combined with immunotherapy. In terms of PFS, there has been an improvement of around 3 months, and the one-year PFS rate has reached 61%, doubling that of chemotherapy combined with immunotherapy.
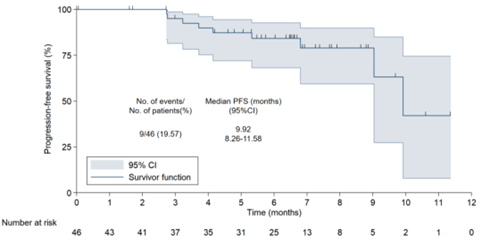
“Figure 2 Progression-Free Survival (PFS) in 46 Patients Treated with Anlotinib in Combination with TQB2450”
We can see that this “chemo-free targeted immunotherapy” regimen has indeed provided different degrees of benefit to patients. Furthermore, the incidence of Grade 3 or higher toxic side effects is only 24%, significantly reducing patients’ adverse reactions compared to chemotherapy combined with immunotherapy and enhancing patient tolerance and treatment completion.
“Table 1 Safety of Anlotinib in Combination with TQB2450”
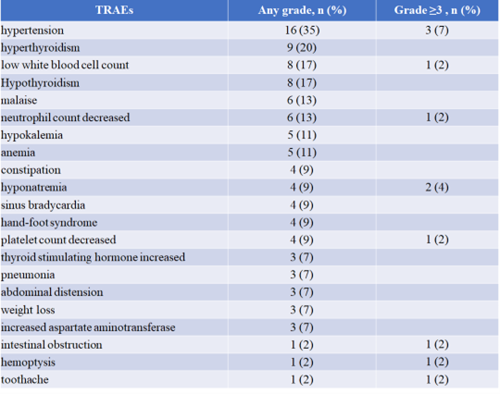
Currently, I believe this study is promising, and we need to conduct large-sample Phase II-III clinical studies to confirm its further efficacy and adverse effects.
Oncology Frontier: Another study exploring neoadjuvant chemotherapy in combination with Trastuzumab, Pembrolizumab, and Nivolumab for HER2-positive resectable gastric/gastroesophageal junction cancer has also garnered significant attention. Could you please introduce the main content and clinical significance of this study as well?
Professor Feng Wang: Gastric cancer is also a significant cancer type in China, with more than 40% of new cases worldwide each year. In China, many gastric cancer patients are already in advanced stages, and they exhibit a high degree of heterogeneity. Among them, there is a subgroup accounting for approximately 10% to 20% with HER2-positive gastric cancer. This subgroup has poor biological behavior, high invasiveness, and a high rate of metastasis, leading to a very poor prognosis for patients.
For HER2-positive gastric cancer, the clinical application of anti-HER2 therapy has brought about a considerable breakthrough. Trastuzumab was the first anti-HER2 drug approved for use in gastric cancer patients, significantly extending the overall survival and progression-free survival of patients. For locally advanced gastric cancer, surgery remains the primary treatment, with neoadjuvant and adjuvant therapy playing significant roles. Historically, neoadjuvant chemotherapy for gastric cancer had a very limited pathological complete response (pCR) rate. With the application of anti-HER2 therapy on top of chemotherapy, the pCR rate increased, ranging from 9% to 23%. However, there is still significant room for improvement in the treatment effectiveness for these patients, and it currently doesn’t meet clinical treatment needs.
The era of immunotherapy has brought about the prominence of immune checkpoint inhibitors, particularly in gastric cancer. Several large randomized controlled trials have established the status of chemotherapy combined with immunotherapy as the first-line treatment for gastric cancer. Especially for HER2-positive gastric cancer patients, the KEYNOTE-811 study has demonstrated that adding Pembrolizumab to Trastuzumab and chemotherapy further enhances patient effectiveness. The overall response rate (ORR) in the Pembrolizumab+Trastuzumab+chemotherapy group reached 74.4%, which is an increase of 22.7% compared to the placebo+Trastuzumab+chemotherapy group. Applying such a four-drug combination regimen to locally advanced patients is expected to achieve better tumor regression, improve the pCR rate, and increase the rate of R0 resection. Based on this background, we have introduced a three-drug combination treatment regimen that includes chemotherapy, Trastuzumab, and domestically produced Tislelizumab, which is referred to as the “targeted immunotherapy chemo-free” approach.
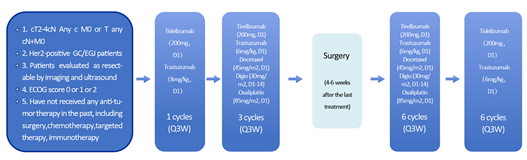
“Figure 3 Design of the ‘Targeted Immuno-Chemotherapy’ Three-Drug Combination Trial”
Currently, we have enrolled over 20 patients in a neoadjuvant treatment-related Phase II clinical trial, and 12 of them achieved excellent outcomes after surgery. The pCR rate reached 58.3%, and the major pathological response (MPR) rate reached 66.7%. Additionally, the tumor regression rate reached 83.3%. Therefore, the short-term efficacy of this regimen is very promising, significantly improving the pCR rate compared to previously reported rates. Of course, our sample size is still relatively small, and we look forward to expanding the number of cases in the trial.
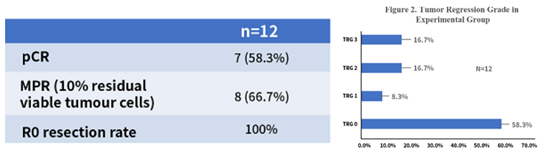
“Figure 4 Short-Term Efficacy of the Three-Drug Combination Regimen”
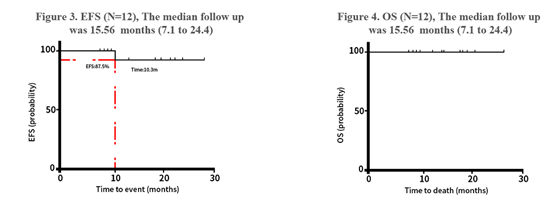
“Figure 5 Event-Free Survival (EFS) and Overall Survival (OS) of the Three-Drug Combination Regimen”
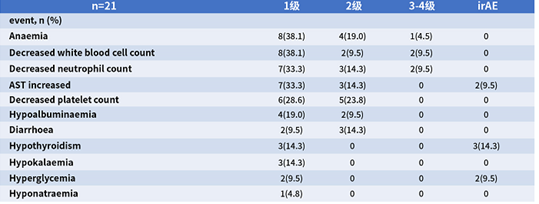
In terms of safety, we can see that the toxic side effects in this regimen are manageable, and no treatment-related deaths occurred. The immune-related adverse events were all Grade 1, so we have high expectations for this regimen.
“Table 2 Safety Events of the Three-Drug Combination Regimen”
Oncology Frontier: Your team has achieved remarkable results in recent years, with numerous successes and multiple presentations at important international academic conferences. What do you believe is the reason behind these outstanding achievements?
Professor Feng Wang:
The main research focus of our team is on clinical research in esophageal and gastric cancer. In recent years, some of our clinical studies have been published at international conferences. I think these achievements can be attributed to two main factors. Firstly, they are the result of the overall development of China’s healthcare system. The increasing number of innovative pharmaceutical companies in China has brought about many innovative drugs, creating conditions for clinical trials. Secondly, it is the result of the efforts and explorations of clinical researchers themselves. Currently, cancer treatment still faces significant challenges, and the current treatment level is relatively low. As a clinical physician, I am faced with the helplessness of not being able to stop the progression of cancer in many patients. I sincerely hope that through the research of our team, we can provide patients with better treatment options. However, continuous effort and innovation are needed. We look forward to sharing more research data with everyone in the future.

Professor Feng Wang
Department of Oncology, First Affiliated Hospital of Zhengzhou University
Director of Ward 1, Oncology Department, First Affiliated Hospital of Zhengzhou University
Chief Physician, Professor, Doctoral Supervisor
Talent Leader in Health Science and Technology of Henan Province
Visiting Scholar at Georgetown University, USA
Leading Talent in Health Science and Technology of Henan Province
Vice Chief Editor of the Journal “Esophageal Diseases”
Outstanding Contribution Award (Esophageal Cancer field) of the “People’s Good Doctor – Jinshan Camellia Plan” in 2020
Director of the Chinese Division of the International Esophageal Disease Association (CSDE)
Director of the Chinese Society of Clinical Oncology (CSCO)
Member of the Esophageal Cancer and Stomach Cancer Special Committee of the Chinese Society of Clinical Oncology (CSCO)
Member of the Liver Cancer and Pancreatic Cancer Special Committee of the Chinese Society of Clinical Oncology (CSCO)
Expert Committee Member of Esophageal Cancer Quality Control of the National Cancer Center
Council Member of the Chinese Anti-Cancer Association Esophageal Cancer, Sarcoma, and International Medical Exchange Special Committee
Vice Chairman of the Beijing Cancer Prevention and Treatment Association Gastric Cancer and Stomach Cancer Special Committee
Standing Committee Member of the Henan Medical Association Oncology Branch
Standing Committee Member of the Henan Anti-Cancer Association
Chairman of the Henan Anti-Cancer Association Sarcoma Special Committee
Standing Committee Member and Youth Committee Chairman of the Henan Anti-Cancer Association
Chairman of the Henan Anti-Cancer Association Esophageal Cancer Special Committee
Director of the Henan Provincial Laboratory for Immune Resistance and Transformation Research in Digestive Tract Tumors


