
In a significant advancement in cancer research, Dr. Yongmei Yin and her team from The First Affiliated Hospital with Nanjing Medical University, Jiangsu Province Hospital published the article "Efficacy, Safety, and Population Pharmacokinetics of MW032 Compared With Denosumab for Solid Tumor–Related Bone Metastases: A Randomized Double-Blind Phase 3 Equivalence Trial" in JAMA Oncology on April, 2024. This pivotal study investigates the efficacy and safety of MW032, a proposed denosumab biosimilar, in treating solid tumor-related bone metastases. Bone metastases, a common and severe complication in cancer patients, lead to skeletal-related events (SREs) that significantly impact morbidity and mortality. The study addresses the urgent need for more effective and accessible treatments for patients suffering from these severe complications.The study aimed to establish the clinical equivalence of MW032 to denosumab, a monoclonal antibody used to prevent SREs in cancer patients with bone metastases. This randomized, double-blind, placebo-controlled phase III trial was conducted across 46 clinical sites in China, involving 701 evaluable patients. The primary endpoint was the percentage change from baseline to week 13 in the natural logarithmic transformed urinary N-telopeptide/creatinine ratio (uNTx/uCr), a biomarker for bone resorption.
The trial enrolled 708 patients, randomly assigned to receive either MW032 or denosumab subcutaneously every four weeks for 53 weeks. The primary efficacy endpoint was the percentage change in uNTx/uCr from baseline to week 13, with secondary endpoints including changes in bone-specific alkaline phosphatase (s-BALP) and the incidence of SREs.
Key findings from the study include:
- Primary Endpoint: The mean change of uNTx/uCr from baseline to week 13 was -72.0% (95% CI -73.5% to -70.4%) in the MW032 group and -72.7% (95% CI -74.2% to -71.2%) in the denosumab group. The mean logarithmic ratios were -1.27 and -1.30, respectively, with a difference of 0.02. The 90% CI for the difference (-0.04 to 0.09) was within the equivalence margin (-0.13 to 0.13), indicating bioequivalence.
- Secondary Endpoints: The differences in uNTx/uCr change at various weeks (5, 25, 37, and 53) were within the equivalence margins. The mean changes in s-BALP were also similar between the groups at all time points during the 53-week period.
- Incidence of SREs: No significant differences were observed in the incidence of SREs or time to first on-study SRE between the MW032 and denosumab groups.
- Safety: The incidence of treatment-emergent adverse events (TEAEs) was similar between the two groups, with 97.2% in the MW032 group and 96.6% in the denosumab group. Grade 3 or higher TEAEs occurred in 53.8% of the MW032 group and 57.6% of the denosumab group. The most common treatment-related TEAEs were hypocalcemia and hypophosphatemia.
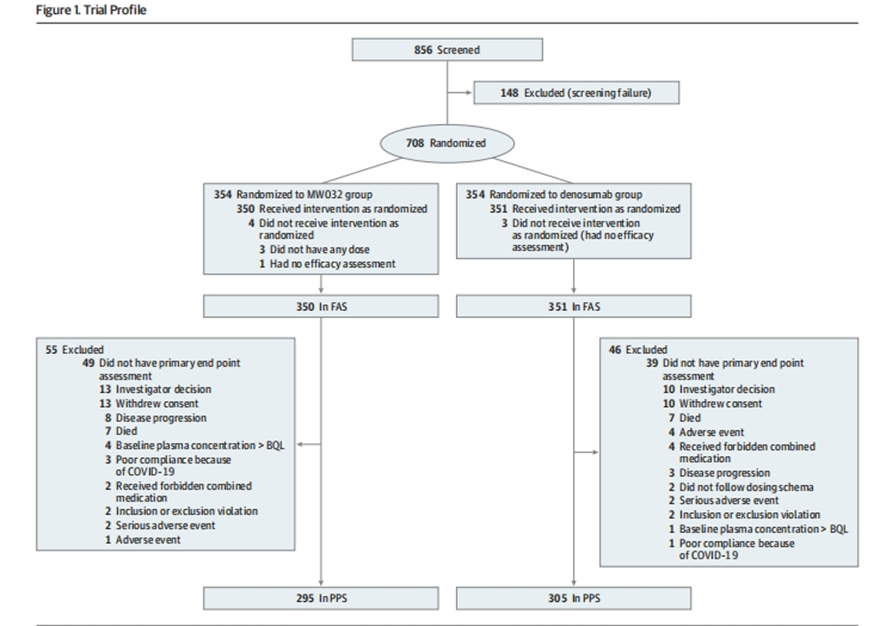
( JAMA Oncol . 2024 Apr 1;10(4):448-455. doi: 10.1001/jamaoncol.2023.6520.)
The trial’s results demonstrated the efficacy and safety of MW032 compared to denosumab:
- Primary Endpoint: The mean change of uNTx/uCr from baseline to week 13 was -72.0% (95% CI -73.5% to -70.4%) in the MW032 group and -72.7% (95% CI -74.2% to -71.2%) in the denosumab group. These percent changes corresponded to mean logarithmic ratios of -1.27 and -1.30, respectively, with a difference of 0.02. The 90% CI for the difference (-0.04 to 0.09) was within the equivalence margin (-0.13 to 0.13).
- Secondary Endpoints: The differences in uNTx/uCr change were 0.015 (95% CI -0.06 to 0.09), -0.02 (95% CI -0.09 to 0.06), -0.05 (95% CI -0.13 to 0.03), and 0.001 (95% CI -0.10 to 0.10) at weeks 5, 25, 37, and 53, respectively. The changes in s-BALP at each time point were also similar, with differences of -0.006 (95% CI -0.06 to 0.05), 0.00 (95% CI -0.07 to 0.07), -0.085 (95% CI -0.18 to 0.01), -0.09 (95% CI -0.20 to 0.02), and -0.13 (95% CI -0.27 to 0.004) at weeks 5, 13, 25, 37, and 53, respectively.
- Incidence of SREs: There was no significant difference in the incidence of skeletal-related events between the two groups, with 32 patients in the MW032 group and 37 patients in the denosumab group experiencing SREs (difference of -1.4%; 95% CI -5.8% to 3.0%).
- Safety: The overall safety profile of MW032 was comparable to that of denosumab, with similar incidences of treatment-emergent adverse events (TEAEs). Grade 3 or higher TEAEs were reported in 53.8% of the MW032 group and 57.6% of the denosumab group. The most common TEAEs included hypocalcemia, hypophosphatemia, and hyperuricemia, with no new safety signals identified.
The study’s significance lies in its demonstration that MW032 is bioequivalent to denosumab in terms of efficacy, safety, and population pharmacokinetics. This biosimilar offers a promising alternative to denosumab, potentially increasing access to effective treatment for patients with solid tumor-related bone metastases.
Conclusion
The phase III equivalence trial provides robust evidence supporting the bioequivalence of MW032 to denosumab in treating solid tumor-related bone metastases. The significant findings in efficacy and safety highlight the potential of MW032 to be a cost-effective alternative, broadening access to crucial treatments and reducing the economic burden on patients. These results are pivotal in shaping future treatment protocols and ensuring that patients with advanced tumors have access to effective and affordable therapies.


