
Editor’s Note: In recent years, the quest for shorter treatment durations and more effective drug combinations to treat drug-sensitive tuberculosis (DS-TB) has become a major research trend in the field of tuberculosis. The aim is to reduce adverse reactions, improve treatment compliance, and enhance treatment efficiency and resource utilization. Studies such as Study 31/A5349, SHINE, and TRUNCATE-TB have already been reported. At the recently concluded 31st Conference on Retroviruses and Opportunistic Infections (CROI 2024), a phase IIc study (abstract number: 164), which was selected for an oral presentation as a late-breaking abstract, explored the efficacy and safety of a 3-month regimen containing clofazimine and rifapentine for treating DS-TB. Infectious Disease Frontier has invited a team led by Dr. Shuihua Lu from The Third People’s Hospital of Shenzhen to introduce and comment on this study as follows:
Study Summary
Abstract Number: 164
Provisional Results From a 3-month Clofazimine/Rifapentine Containing Regimen for Drug-Sensitive TB
Background:
CLO-FAST (ACTG A5362; NCT04311502) is a randomized, controlled, open-label phase IIc clinical trial aimed at evaluating the safety and efficacy of a 3-month treatment regimen of rifapentine (P, 1200mg daily)/clofazimine (CFZ, 300mg daily for the first three weeks, then 100mg) (8w HPZEC/ 5w HPZC) compared to a 6-month standard treatment regimen (SOC) for drug-sensitive (DS) tuberculosis. The study was stopped early due to a lack of clinical efficacy. This report presents data results up to September 25, 2023, when the last participant completed the study treatment.
Methods:
Adult DS patients were recruited at six sites in Malawi, Zimbabwe, South Africa, India, and Haiti. Participants were randomized and stratified based on HIV status and the presence of advanced disease in chest X-rays, into: Group 1 (13-week experimental regimen) receiving 3PHZEC; Group 2 (26-week SOC) receiving 2RHZE/4RH; and Group C (a PK sub-group without CFZ loading, then SOC) receiving 1PHZEC/1RHZE/4RH. The primary outcomes of the study were the time to stable culture conversion at week 12 (efficacy) and the proportion of participants experiencing ≥grade 3 adverse events (AEs) at week 65 (safety). The key secondary outcomes were the proportions of participants in Groups 1 and 2 experiencing adverse clinical/bacteriological outcomes at week 65.
Results:
Between June 2021 and April 2023, when the trial was terminated early based on DSMB recommendations due to inefficacy, 58 of the planned 110 participants were included in Group 1, 31 of the planned 55 participants were included in Group 2, and 15 of the planned 20 participants were included in Group C. The majority of participants were male (79%), with 29% being HIV-positive (median baseline CD4+ cell count 265 cells/mm3, IQR 185-379 cells/mm3). Seventy-one percent of patients had radiologically advanced tuberculosis, and 25% were 3+ smear-positive at enrollment. The median study follow-up time (IQR) was 50.2 weeks (39.7, 57.6 weeks). By week 12, 89% (n=48) of participants in Group 1 and 90% (n=28) of participants in Group 2 achieved stable culture conversion (adjusted HR 1.17, 90%CI: 0.79 to 1.73, adjusted for baseline HIV status and presence of advanced disease). Influenced by changes in creatinine clearance rates in Group 1, the cumulative proportion of participants experiencing ≥grade 3 adverse events was higher in Group 1 (46%) than in Group 2 (16%) (difference 30%, 90%CI: 14-45%). The cumulative incidence rates of adverse outcomes for participants in Groups 1 and 2 were 49% (95%CI: 34-67%) and 34% (95%CI: 19-58%), respectively (difference -15%, 95%CI: -41% to 11%). The table below describes the clinical/bacteriological adverse outcome events.
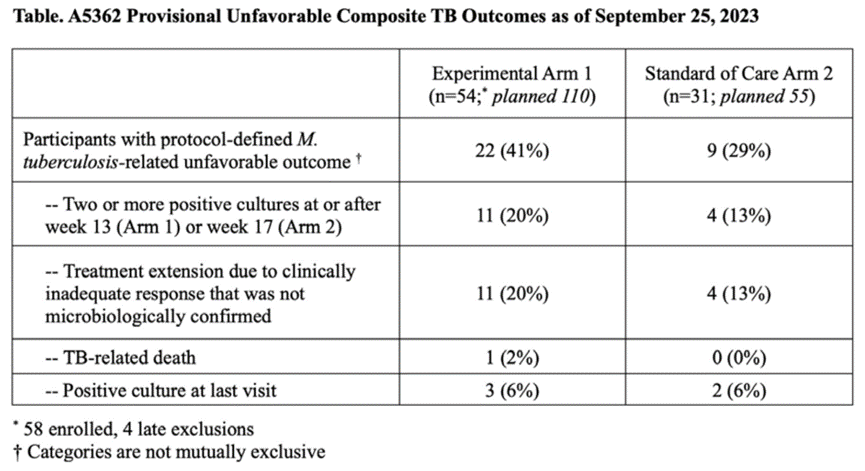
Conclusion:
The 13-week treatment regimen containing CFZ and rifapentine is insufficiently effective for DS-TB. Despite a high rate of culture conversion by week 12, the SOC group exhibited a higher rate of adverse events.
Study Commentary
A major trend in tuberculosis research in recent years has been the exploration of shorter treatment courses and more effective drug combinations for drug-sensitive tuberculosis (DS-TB). The key drivers and objectives behind this trend include: (1) Enhancing patient compliance: The standard treatment regimen (HRZE) typically lasts six months or longer, posing a significant burden on patients. This can lead to treatment interruption or incompleteness, potentially resulting in treatment failure and the development of drug resistance. Short-course treatments aim to alleviate patient burdens and increase treatment completion rates. (2) Reducing adverse reactions: Long-term use of anti-tuberculosis drugs can increase the risk of adverse reactions, especially side effects such as hepatotoxicity. Short-course treatments could mitigate these risks. (3) Improving treatment efficiency and resource utilization: Short-course treatments can enhance the efficiency of medical resource use, which is especially significant for public health improvements in resource-limited settings.
Successful examples of short-course treatment studies include: (1) Study 31/A5349, which reduced the treatment duration to four months by substituting high-dose rifapentine for rifampicin and moxifloxacin for ethambutol. (2) The SHINE study, which shortened the standard course to four months for children and adolescents with milder conditions. (3) The TRUNCATE-TB study, which utilized bedaquiline and linezolid to replace rifampicin for two months of intensified treatment, or adjusted to three months based on treatment response, with a switch to standard treatment if ineffective. This strategy shortened the total treatment duration to 86 days. These three studies, published in 2021, 2022, and 2023, respectively, represent significant advancements in short-course treatment.
The Clo-Fast study, conducted concurrently, sought to explore a new treatment regimen by adding clofazimine to the standard regimen and substituting rifapentine for rifampicin at a higher dose. Clofazimine, originally used for treating leprosy, has gained prominence in anti-tuberculosis treatment in recent years. It works by interfering with the cell membrane and electron transport chain of Mycobacterium tuberculosis; it is effective against multidrug-resistant and extensively drug-resistant tuberculosis strains and is one of the WHO-recommended drugs for treating multidrug-resistant tuberculosis. Its main side effects include skin discoloration, gastrointestinal discomfort, and QT interval prolongation. The anti-tuberculosis effect of rifapentine, especially at higher doses, has also received attention in recent years, with many studies suggesting the use of higher doses for potentially more effective short-course treatments.
However, the Clo-Fast study did not achieve success, which may be attributed to:
(1) Insufficient efficacy of the drug regimen: The 3-month regimen (including high-dose rifapentine and clofazimine) failed to achieve the same treatment effects as the 6-month standard treatment regimen. Although the stable culture conversion rates were similar at 12 weeks, the efficacy of the short-course treatment for DS-TB was inadequate.
Conclusion:
The 13-week treatment regimen containing clofazimine and rifapentine showed poor efficacy in treating DS-TB. Despite a high rate of culture conversion at week 12, the experimental group also experienced a higher incidence of adverse events compared to the standard of care group.
Study Commentary
In recent years, one of the major trends in tuberculosis research has been the exploration of shorter treatment durations and more effective drug combinations for treating drug-sensitive tuberculosis (DS-TB). The key motivations and goals behind this trend include: (1) Enhancing patient compliance: The standard treatment regimen (HRZE) typically lasts six months or longer, posing a significant burden on patients and leading to treatment interruptions or incompleteness, which could result in treatment failure and the development of resistance. Shorter treatment courses aim to alleviate patient burden and improve treatment completion rates. (2) Reducing adverse reactions: Long-term use of anti-tuberculosis drugs may increase the risk of adverse reactions, especially side effects like hepatotoxicity. Shorter treatment courses are hoped to mitigate these risks. (3) Improving treatment efficiency and resource utilization: Short-course treatments could lead to more efficient use of medical resources, which is especially significant for public health improvements in resource-limited settings.
Successful examples of short-course treatment studies include: (1) Study 31/A5349, which shortened the treatment duration to 4 months by substituting high-dose rifapentine for rifampin and moxifloxacin for ethambutol. (2) The SHINE study, which shortened the standard regimen to 4 months for children and adolescents with milder conditions. (3) The TRUNCATE-TB study, which involved 2 months of intensified treatment with bedaquiline and linezolid replacing rifampin, or adjusted to 3 months based on treatment response, with a shift to standard treatment if the response was inadequate, shortening the total treatment duration to 86 days. These studies, published in 2021, 2022, and 2023, respectively, represent significant advancements in short-course treatment.
The Clo-Fast study, conducted concurrently, sought to explore a new treatment regimen by adding clofazimine to the standard regimen and replacing rifampin with a high dose of rifapentine. Clofazimine, originally used for treating leprosy, has gained importance in anti-tuberculosis treatment in recent years by interfering with the cell membrane and electron transport chain of Mycobacterium tuberculosis; it is effective against multidrug-resistant and extensively drug-resistant TB strains and is one of the WHO-recommended drugs for treating multidrug-resistant TB. Its main side effects include skin discoloration, gastrointestinal discomfort, and QT interval prolongation. Recently, the anti-tuberculosis effect of rifapentine has also received attention, with many studies suggesting the use of higher doses. Higher doses of rifapentine may be more effective in short-course treatments.
However, the Clo-Fast study was not successful, possibly due to:
(2) Higher incidence of adverse events: The experimental group experienced a higher proportion of adverse events (especially those related to changes in creatinine clearance rate) than the standard treatment group, possibly indicating that the short-course regimen’s safety was inferior to the standard treatment, affecting the overall treatment effect. Clofazimine is mainly excreted through feces, with only a small portion excreted through urine, and its excretion is not significantly affected in patients with reduced kidney function, usually not requiring dose adjustment. The reasons for kidney-related adverse events in the experimental group require further analysis, and caution is needed regarding renal toxicity brought by high doses of rifapentine or drug interactions within the regimen.
(3) Higher probability of adverse outcomes: The experimental group’s probability of adverse outcomes (clinical/bacteriological) was higher than that of the standard treatment group, possibly because the short-course treatment failed to completely clear the infection or effectively prevent relapse. Compared to the TRUNCATE-TB study, the Clo-Fast study’s treatment regimen might have been less effective; the former used two WHO Group A recommended drugs (i.e., bedaquiline and linezolid, without using rifampicin), while the latter only used one WHO Group B recommended drug (i.e., clofazimine) and high-dose rifapentine.
(4) Insufficient sample size and early termination: The study was terminated early due to not achieving the expected effects, with the number of participants not reaching the planned sample size, potentially limiting the statistical power and reliability of the study results.
(5) Diversity of population and disease conditions: The study spanned different countries, with participants having varying demographic characteristics and disease states (such as HIV infection and severity of tuberculosis), which could affect treatment responses and the incidence of adverse events.
In summary, the Clo-Fast study was innovative in attempting to shorten the treatment duration for DS-TB, but ultimately it did not achieve the expected efficacy and safety targets.

Dr. Shuihua Lu
Deputy Director of the National Clinical Research Center for Infectious Diseases
Director of the Pulmonary Medicine Department, The Third People’s Hospital of Shenzhen

Liang Fu
National Clinical Research Center for Infectious Diseases
The Second Department of Pulmonary Diseases, The Third People’s Hospital of Shenzhen


