Editor’s Note:This 2023 ESMO Annual Meeting, in the field of gastric cancer, the global Phase III MATTERHORN study of the anti-PD-L1 antibody pembrolizumab in combination with the FLOT regimen for neoadjuvant treatment in resectable gastric and gastroesophageal junction adenocarcinoma (GC/GEJC) was presented as a Late-breaking Abstract (LBA) with mid-term analysis results (Abstract No.: LBA73). The mid-term analysis showed a clinically and statistically significant improvement in the pathological complete response (pCR) rate, making MATTERHORN the first neoadjuvant Phase III immunotherapy regimen in gastric cancer to show positive results. “Tumor Outlook” invited Professor Liang Han from Tianjin Medical University Cancer Hospital to provide an in-depth interpretation of the study.
MATTERHORN Study (Abstract No.: LBA73) [1]
The MATTERHORN study (NCT04592913) is a global, randomized, double-blind, placebo-controlled Phase III clinical trial designed to evaluate the efficacy and safety of pembrolizumab in combination with FLOT for neoadjuvant treatment in resectable GC/GEJC patients. Here, we report the pre-specified mid-term analysis (IA) results.
Patients with resectable (>T2N0-3M0/T0-4N1-3M0) GC/GEJC were randomly assigned (1:1) to the pembrolizumab group or placebo group. Patients in the pembrolizumab group received two cycles of pembrolizumab (1500mg, D1, Q4W) or placebo in combination with FLOT (D1/D15, Q4W) both preoperatively and postoperatively, followed by 10 cycles of adjuvant pembrolizumab or placebo (D1, Q4W). The primary endpoint of the study is event-free survival (EFS) in the intention-to-treat (ITT) population, with key secondary endpoints including pCR and overall survival (OS). Pre-specified IA was performed in all randomized patients who underwent surgery or were excluded, and pCR rates were assessed by central review (modified Ryan) for superiority (two-sided α=0.1%) and safety and surgical outcomes were evaluated.
Baseline Characteristics
Both arms included 474 patients, and baseline characteristics were balanced between the two groups. Asian patients accounted for 19%, with 66% in clinical stage T3, 25% in T4, and 70% with positive lymph nodes (cN+).
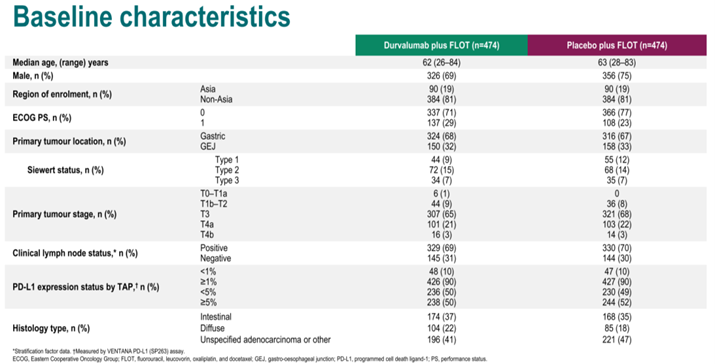
Baseline Characteristics of the MATTERHORN Study
Study Results
Compared to the placebo group, the pembrolizumab in combination with FLOT group showed a statistically significant improvement in pCR (19% vs. 7%; Δ12%; OR 3.08, P<0.00001). The pCR rate and near pCR (pnCR) rate in the pembrolizumab group were 27%, nearly double that of the control group (14%).
The surgical resection rates in the pembrolizumab group and placebo group were 87% and 84%, respectively. In patients who underwent surgery, both groups had an R0 resection rate of 86%, but the pembrolizumab group had a higher rate of downstaging compared to the placebo group (pT0: 23% vs. 11%; pN0: 52% vs. 37%).
Median exposure times were similar in both groups. The rates of grade 3/4 adverse events were similar, with 69% in the pembrolizumab group and 68% in the placebo group.
Dr. Liang’s Commentary
Locally advanced gastric cancer (LAGC) refers to cancer tissue invading the inherent muscle layer of the stomach wall or penetrating the muscle layer to reach the serosa (clinical staging cT1-2N+M0 or cT3-4bNanyM0). In China, LAGC accounts for as high as 60% of cases [2], and the 5-year survival rate still needs improvement, with 5-year OS rates dropping from 81.9% in stage II to 18.4% in stage III [3]. Although radical surgery is still an important means for patients to achieve a cure, in GC/GEJC patients who are eligible for surgical treatment, the recurrence rate after D2 radical surgery and postoperative chemotherapy can still be as high as 50% to 80% [4]. Therefore, surgery combined with neoadjuvant treatment is an important means to reduce recurrence and improve survival [5]. Currently, neoadjuvant treatment for GC/GEJC mainly consists of chemotherapy. The 2023 CSCO Gastric Cancer Guidelines recommend that for patients with cT2 — T4 and cT1N+ who are eligible for surgical resection, D2 lymph node dissection combined with neoadjuvant or adjuvant chemotherapy is recommended, with different priorities depending on the primary tumor location (gastroesophageal junction or non-gastroesophageal junction) and stage (II or III) [6].
After the success of immune checkpoint inhibitors in the advanced GC/GEJC field [7-9], they have also started to be used in neoadjuvant treatment. Several Phase III clinical trials have been initiated, including ATTRACTION-5 (NCT03006705), KEYNOTE-585 (NCT03221426), HLX10-006-GCneo (NCT04139135), and MATTERHORN (NCT04592913).
ATTRACTION-5 is a postoperative adjuvant immunotherapy trial with negative results, as revealed at this year’s ASCO meeting [10]. The study included 755 pIII GC/GEJC patients who underwent D2 or extended radical surgery, randomized (1:1) to receive nivolumab or placebo in combination with SOX/CAPOX adjuvant therapy. The primary endpoint, recurrence-free survival (RFS), showed no significant difference between the nivolumab group and the placebo group (3-year RFS rates: 68.4% vs. 65.3%; HR 0.90, 95% CI: 0.69-1.18; P=0.4363), falling short of the expected 71%.
KEYNOTE-585 is another Phase III trial (LBA74) reported at this year’s ESMO meeting with negative results [11]. The study included four arms (two cohorts): in the first cohort, patients received three cycles of pembrolizumab (200mg q3w) or placebo in combination with chemotherapy (cisplatin plus 5-Fu or capecitabine) before and after surgery, followed by 11 cycles of pembrolizumab or placebo in the adjuvant phase. In the second cohort, the regimen was similar, but the chemotherapy regimen was FLOT. The results showed a significant increase in pCR in the pembrolizumab group compared to the placebo group in the FLOT cohort (13.0% vs. 2.4%, P<0.0001), but the difference in the primary endpoint EFS did not reach the pre-specified statistical significance (45.8 vs. 25.7 months, HR 0.81, P=0.011); the OS in both groups was not significantly different (60.7 months vs. not reached, HR 0.93).
The HLX10-006-GCneo study included patients with clinical stage cT3N+M0, providing a more refined patient population compared to the other three studies. The neoadjuvant treatment regimen in the study consisted of surufatinib (HLX10, an anti-PD-1 antibody) or placebo in combination with SOX in the neoadjuvant phase, followed by surufatinib or SOX in the adjuvant phase, and the study is currently ongoing.
Following the MATTERHORN study, pembrolizumab has also been preliminarily explored in the neoadjuvant treatment of microsatellite-high (MSI-H) gastric cancer. The multicenter, multi-cohort, single-arm Phase II INFINITY study [19] reported at the 2023 ASCO-GI included 18 GC/GEJC patients with dMMR/MSI-H and clinical stage T2-4N0M0, receiving three cycles of pembrolizumab in combination with Tremelimumab (an anti-CTLA-4 antibody) in the neoadjuvant phase, achieving a high pCR rate of up to 60% (9/15).
In summary, the immunotherapy approach for resectable GC/GEJC still needs further exploration. Questions remain about whether patient stratification based on clinical stage and tumor biomarkers (PD-L1, MSI-H) can help identify the optimal treatment strategies (monotherapy or combination therapy). Additionally, the choice of immune checkpoint inhibitors, such as anti-PD-L1 antibodies or anti-PD-1 antibodies, and the selection of chemotherapy regimens are important factors that need further clarification. The MATTERHORN study used an all-comer approach, and the neoadjuvant pembrolizumab in combination with FLOT regimen demonstrated a significant improvement in pCR rates. Moreover, the study included a significant proportion of Asian patients, which may have implications for the treatment of Chinese patients in the future. We look forward to the release of EFS results in the future, which may bring greater benefits to a larger patient population.
References
[1]Y.Y. Janjigian,et al.Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) inresectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. ASCO 2023. LBA 73
[2]Zeng H, Ran X, An L, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6(12):e877-e887. doi:10.1016/S2468-2667(21)00157-2
[3]Wang H, Guo W, Hu Y, et al. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: Comparative analysis of the 7th and 8th editions in a monoinstitutional cohort. Mol Clin Oncol. 2018;9(4):423-431. doi:10.3892/mco.2018.1683
[4]朱正纲. 局部进展期胃癌围手术期治疗的现状与展望[J]. 中华胃肠外科杂志, 2021, 24(2): 101-106.
[5]胃肠肿瘤学:原理与时间/(美)凯尔森(Kelsen DP)等编著;梁寒等译.天津科技翻译出版社.2012.3.P318-321,P330-331
[6]中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)胃癌诊疗指南2023[M]. 北京: 人民卫生出版社, 2023.
[7]Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942-948. doi:10.1038/s41586-022-04508-4
[8]Jianming Xu, Yongshuai Jin, Ying Liu, et al.ORIENT-16: Sintilimab plus XELOX vs placebo plus XELOX as 1st line treatment for unresectable advanced gastric and GEJ adenocarcinoma. Cancer Res 1 July 2019; 79 (13_Supplement): CT213. https://doi.org/10.1158/1538-7445.AM2019-CT213
[9]Sun Young Rha, Lucjan Wyrwicz, Patricio Eduardo Yanez Weber,et al.KEYNOTE-859 study of pembrolizumab plus chemotherapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Outcomes in the protocol-specified PD-L1–selected populations. Journal of Clinical Oncology 41, no. 16_suppl (June 01, 2023) 4014-4014.DOI: 10.1200/JCO.2023.41.16_suppl.4014
[10]Masanori Terashima, et al.ATTRACTION-5: A phase 3 study of nivolumab plus chemotherapy as postoperative adjuvant treatment for pathological stage III (pStage III) gastric or gastroesophageal junction (G/GEJ) cancer.ASCO 2023.Abstract 4000
[11]K. Shitara, et al.Pembrolizumab plus chemotherapy vs chemotherapy as neoadjuvant and adjuvant therapy in locally-advanced gastric and gastroesophageal junction cancer: The phase III KEYNOTE-585 study.ESMO 2023.LBA 74
[12]Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. doi:10.1056/NEJMoa055531
[13]Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387-4393. doi:10.1200/JCO.2011.36.5908
[14]Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389-1396. doi:10.1016/S1470-2045(14)70473-5
[15]Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948-1957. doi:10.1016/S0140-6736(18)32557-1
[16]J.Ji, et al.Perioperative Chemotherapy of Oxaliplatin Combined with S-1 (SOX) versus Postoperative Chemotherapy of SOX or Oxaliplatin with Capecitabine (XELOX) in Locally Advanced Gastric Adenocarcinoma with D2 Gastrectomy: a Randomized Phase III Trial (RESOLVE Trial).ESMO 2019.Abstract 3635
[17]Yoon-Koo Kang, et al.Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel(D), oxaliplatin(O) and S-1(S) (DOS) followed by surgery and adjuvant S-1, vs surgery and adjuvant S-1, for resectable advanced gastric cancer (GC) (PRODIGY).ESMO 2019.LBA41
[18]L. Chen,et al. Early results of the randomized, multicenter, controlled evaluation of S-1 and oxaliplatin as neoadjuvant chemotherapy for Chinese advanced gastric cancer patients (RESONANCE Trial).2020 ASCO-GI, Abstract #284235.
[19]Filippo Pictrantonio, et al.Multicentre, single-arm, multi-cohort, phase ll trial of tremelimumab and durvalumab as neoadjuvanttreatment of patients with microsatellite instability-high (MSl) resectable gastric or gastroesophageal junctionadenocarcinoma(GAC/GEJAC):the INFINITY study by GONO. ASCO-GI 2023,Abstract 358

Professor Liang Han
Professor, Chief Physician
Director, Gastric Cancer Center
Tianjin Medical University Cancer Hospital
TAG: ESMO2023;Gastric cancer;GC/GEJC;Commentary


