The commentary “Alpha-enolase promotes progression of acute myeloid leukemia via MAPK/ERK signaling pathway” by Zhang et al., published in Blood Science (2025), examines the clinical and biological significance of ENO1 in acute myeloid leukemia (AML). ENO1, a glycolytic enzyme with additional regulatory roles, has been implicated in several solid tumors, but its function in AML had not been fully clarified. Through integrated analyses of patient samples, functional assays, transcriptomics, and in vivo models, the authors demonstrate that ENO1 is overexpressed in AML and promotes disease progression mainly through MAPK/ERK pathway activation.
Clinical Expression Patterns and Prognostic Value of ENO1
Multiple datasets—including TCGA, BeatAML, and LeuceGene—showed consistently elevated ENO1 expression in AML bone marrow compared with healthy donors. In an in-house cohort of 185 AML patients and 61 controls, ENO1 was significantly higher (p = 0.003). High ENO1 correlated with intermediate-to-poor molecular risk groups and predicted inferior overall and disease-free survival, with hazard ratios close to 2.
ENO1 expression was further elevated in refractory and non-remission samples, suggesting a link to chemoresistance. Across FAB subtypes, ENO1 overexpression appeared broadly distributed, underscoring its relevance across different AML phenotypes.
ENO1 Knockdown Impairs Leukemic Growth and Survival
ENO1 knockdown in KG-1, U937, and OCI-AML3 cells produced strong functional effects. ENO1 mRNA decreased by 70–90% and protein levels by 50–80%. In CCK8 assays, shENO1 U937 cells showed almost no proliferation over 48–96 hours, whereas control cells grew steadily; similar 80% reductions were observed in the other cell lines.
Clonogenic assays showed sharply reduced colony number and size after ENO1 depletion. Migration decreased by approximately 50–70%, while invasion fell by 60–80%. Cell-cycle analyses demonstrated robust G1 arrest with reduced S-phase entry, and apoptosis increased significantly. Together, these findings confirm that ENO1 supports AML cell growth, survival, and invasiveness.
(Blood Science. 7(3):e00246, September 2025.)
Transcriptomic Evidence Linking ENO1 to MAPK/ERK Regulation
RNA-seq comparing U937 and shENO1 U937 cells identified 385 upregulated and 389 downregulated genes. KEGG pathway enrichment pointed strongly toward MAPK signaling alterations, while GSEA showed significant enrichment for negative regulation of MAP kinase activity (NES = –1.876, p < 0.001). Western blotting validated these signals, showing substantially reduced phosphorylation of Raf, MEK, and ERK in ENO1-knockdown cells, with total protein levels unaffected. These data position ENO1 upstream of MAPK/ERK signaling in AML.
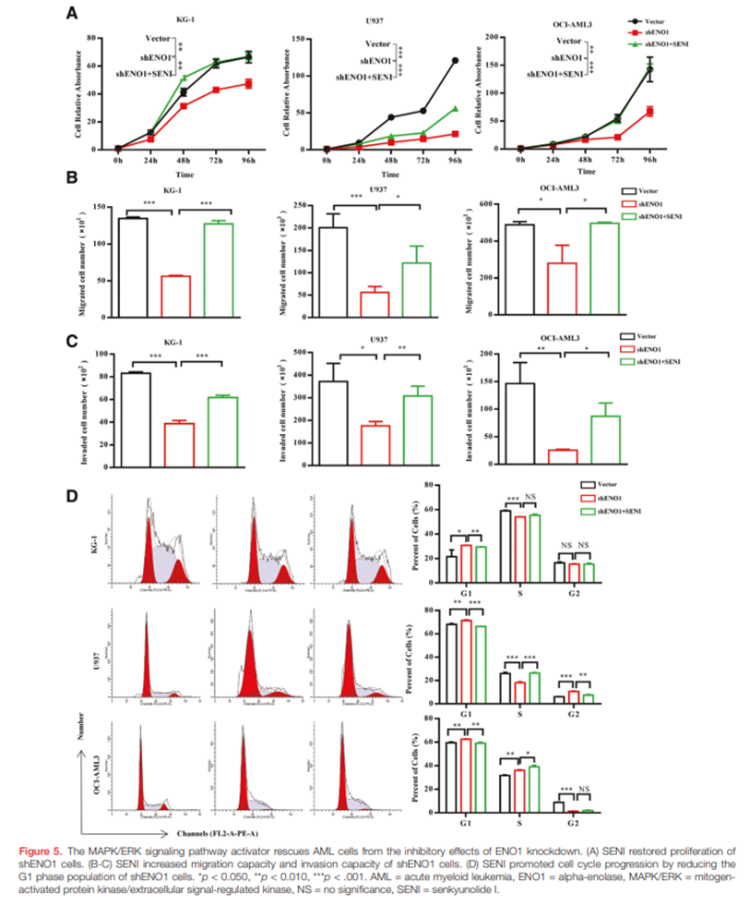
Rescue of ENO1 Loss by ERK Pathway Activation
To test whether ERK signaling mediates ENO1 function, researchers treated shENO1 cells with SENI, an ERK pathway activator. SENI restored proliferation in all three AML cell lines, returning growth curves nearly to control levels. It also reversed migration and invasion defects, and normalized the cell cycle by reducing G1 accumulation and restoring S-phase entry. These rescue experiments confirm that ERK activation can compensate for ENO1 loss.
MEK Inhibition Mimics ENO1 Knockdown
Treatment with U0126, a MEK inhibitor, produced effects similar to ENO1 depletion. Proliferation was greatly suppressed from 24 to 96 hours, colony formation decreased markedly, and migration and invasion declined sharply. U0126 induced G1 arrest and increased apoptosis, mirroring shENO1 findings. Western blots confirmed reduced phosphorylation of MEK and ERK. These results reinforce that ENO1-driven leukemic progression depends on MAPK/ERK signaling.
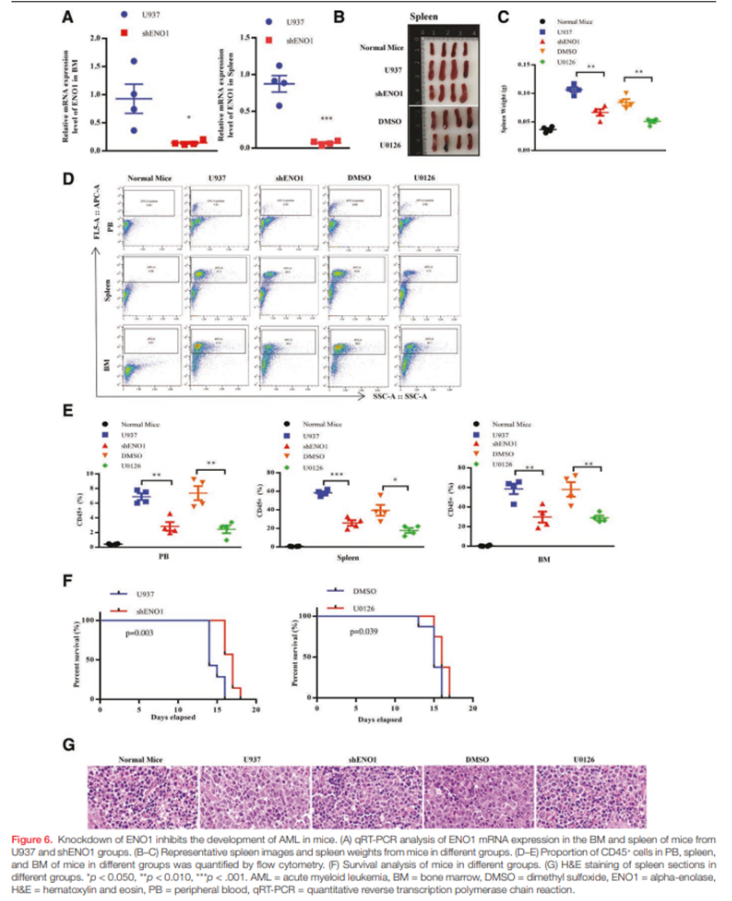
In Vivo Validation of ENO1’s Oncogenic Role
In xenograft models, shENO1 U937 cells produced significantly less leukemia burden. Leukemic infiltration in peripheral blood, spleen, and bone marrow was markedly reduced. Spleen weights decreased by about 37% in shENO1 mice compared with controls. U0126 produced a similar reduction of roughly 39%. Flow cytometry showed reduced CD45⁺ leukemic cells in all treated groups, while H&E staining demonstrated less blast infiltration. Survival was significantly prolonged in both shENO1 mice (p = 0.003) and U0126-treated mice (p = 0.039).
(Blood Science. 7(3):e00246, September 2025.)
Conclusion
Zhang et al. identify ENO1 as a clinically meaningful oncogene in AML. By sustaining MAPK/ERK activation, ENO1 promotes proliferation, invasion, and leukemic survival. Its strong prognostic relevance and central signaling role make ENO1 a promising therapeutic target. Combining ENO1 inhibition with MAPK/ERK pathway blockade may offer an effective strategy for high-risk or relapsed AML.
Click the link to view the original article:
https://journals.lww.com/bls/fulltext/2025/09000/alpha_enolase_promotes_progression_of_acute.8.aspx



