Editor's Note: The annual American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO-GU) was recently held in San Francisco, USA. Over its twenty-year history, the ASCO-GU Symposium has witnessed the evolution of precision treatment for genitourinary cancers. However, the strategies for precision treatment vary significantly among prostate cancer, kidney cancer, and urothelial carcinoma. In an interview with Oncology Frontier, Dr. Yige Bao from West China Hospital, Sichuan University, focused on the treatment strategies for metastatic castration-resistant prostate cancer (mCRPC) based on genetic testing to guide PARP inhibitor (PARPi) therapy. He also shared a study on neoadjuvant therapy for upper tract urothelial carcinoma (UTUC) that his team submitted to the ASCO-GU Symposium.
Oncology Frontier: Can you share with us the research your team submitted to this ASCO-GU meeting?
Dr. Yige Bao: At this ASCO-GU meeting, I represented the urology team led by Professor Qiang Wei from West China Hospital to report on an ongoing phase II clinical trial initiated by researchers. This study aims to explore the efficacy and safety of neoadjuvant therapy using the immune checkpoint inhibitor (ICI) tislelizumab and the antibody-drug conjugate (ADC) disitamab vedotin in patients with high-risk or locally advanced upper tract urothelial carcinoma (UTUC), stages cT2-4N0M0 or cT1-4N1-2M0.
A key motivation for initiating this study is that the future treatment for high-risk UTUC patients will tend to neoadjuvant therapy rather than adjuvant therapy. Because most UTUC patients undergo nephroureterectomy, leaving only one kidney, which decreases their tolerance and acceptability to platinum-based chemotherapy. Additionally, UTUC patients are more likely to have kidney function problems, making it difficult to tolerate traditional platinum-based neoadjuvant or adjuvant chemotherapy. Therefore, our study, which uses ADC combined with ICI that has relatively lower side effects compared to chemotherapy, hopes to enable more patients to complete a full course of neoadjuvant therapy. Furthermore, our study does not restrict enrollment to patients positive for HER2 and PD-L1 expression, as preliminary research shows that the majority of UTUC tumors exhibit overexpression of HER2 or PD-L1 immunogenicity, with only a minority of tumors being luminal papillary tumors primarily carrying FGFR2/3 mutations. Hence, we are also looking forward to the data indicating whether the combination of PD-1 inhibitors and HER2-ADC can achieve high efficacy in a population without molecular testing.
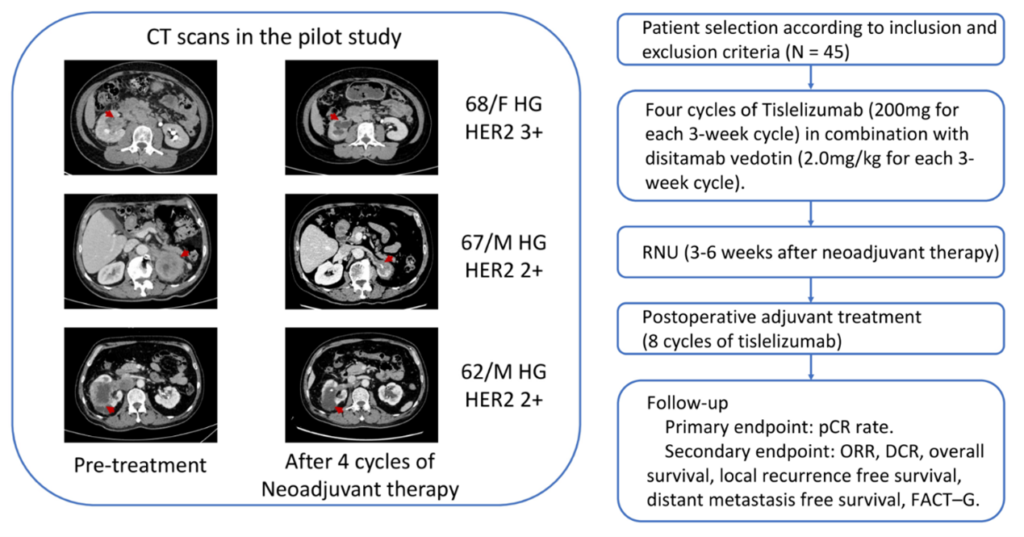
△ Phase II Study Design on the Combination of ICI and ADC for Neoadjuvant Treatment of UTUC Initiated by the Department of Urology, West China Hospital
Oncology Frontier: As you mentioned molecular/genetic testing, we know that in advanced prostate cancer, treatment with PARPi is still guided by testing for mutations in genes like BRCA and other HRR (homologous recombination repair) mutations. The BRCAAway study reported at this ASCO-GU Symposium further analyzes the first-line treatment using either olaparib or abiraterone alone, or in combination. Could you discuss the implications for clinical practice?
Dr. Yige Bao: In the field of metastatic castration-resistant prostate cancer (mCRPC), studies such as PROfound and PROpel have confirmed the benefits of olaparib as a monotherapy in second-line treatment and olaparib combined with abiraterone as a first-line treatment, especially in patients with BRCA1/2 or other HRR gene mutations. Therefore, current guidelines recommend timely genetic testing for patients with advanced prostate cancer to guide the choice of treatment.
The BRCAAway study, presented in the prostate cancer oral session at this ASCO-GU Symposium, is a multi-cohort phase II study exploring different first-line treatment modalities with PARPi or NHA (novel hormonal agent), enrolling patients with treatment-naive mCRPC (who have not received anti-androgen therapy in the mHSPC stage). Patients were randomly divided into four cohorts, with cohorts 1-3 targeting patients with BRCA1/2 and/or ATM mutations: cohort 1 patients were treated with abiraterone, with crossover to olaparib allowed upon progression; cohort 2 received olaparib, with crossover to abiraterone allowed upon progression; cohort 3 was treated with both olaparib and abiraterone; and cohort 4 targeted patients with other non-classical DNA repair defects, receiving olaparib plus abiraterone. The results reported at this ASCO-GU Symposium for cohorts 1-3 showed median PFS (progression-free survival) of 8.4 months, 14 months, and 39 months, respectively, with PSA response rates of 58%, 67%, and 95%, respectively.
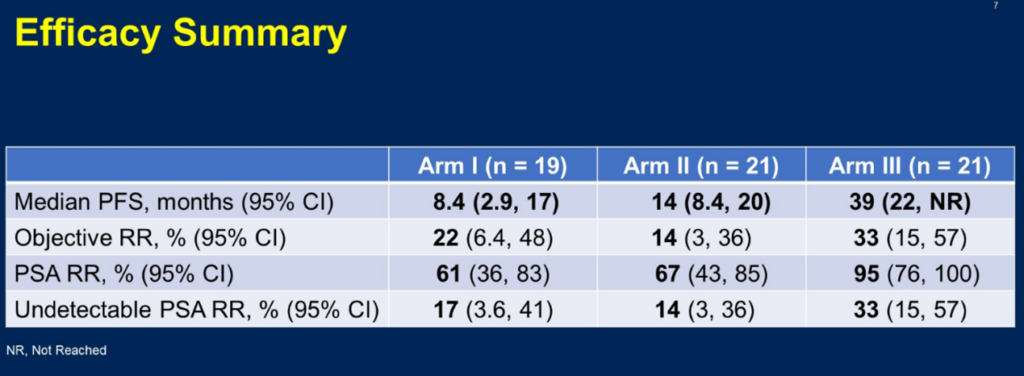
△ Efficacy Summary of the BRCAAway Study
This study offers several key insights: Firstly, patients with BRCA or ATM mutations show a high degree of sensitivity to treatment with olaparib, either as a monotherapy or in combination, with the combination therapy’s median PFS reaching up to 39 months. This makes it the optimal choice for first-line treatment, significantly extending the duration compared to the use of the two drugs separately. However, in clinical practice, some patients cannot tolerate NHA (novel hormonal agents) or afford the high costs associated with combination therapy. Most prostate cancer patients are elderly and may have complex underlying diseases, such as heart failure, volume or electrolyte imbalances, and often cannot tolerate the adrenal glucocorticoid-related adverse reactions caused by abiraterone/prednisone. The BRCAAway study indicates that choosing olaparib as a single agent is viable for patients with HRR mutations, offering efficacy comparable to NHA monotherapy and even longer in duration. Therefore, if genetic testing indicates an HRR mutation, patients should receive olaparib, either as monotherapy or in combination, as early as possible, especially since the survival benefit is most evident with combination therapy.
Oncology Frontier: With precision treatment and testing taking the lead, in your opinion, which patients are the main candidates for PARP inhibitors? How to optimize the use strategy of PARP inhibitors to bring longer benefits to prostate cancer patients?
Dr. Yige Bao: Olaparib and other PARP inhibitors have brought revolutionary changes to the field of prostate cancer treatment, especially for patients carrying BRCA1/2 or other HRR gene mutations. In the PROfound and PROpel studies, we have seen significant efficacy of olaparib in specific patient groups. The PROfound study demonstrated the efficacy of olaparib monotherapy in patients with mCRPC who carry BRCA mutations and have previously failed NHA treatment, achieving a median OS of 20.1 months and a median rPFS of 9.8 months, which is triple that of the control group. The PROpel study is the first phase III study to prove that combining abiraterone with PARPi can bring clinical benefits in first-line treatment for all mCRPC patients without genetic selection. The detailed data for the HRRm population reported at this ASCO-GU conference showed that overall, 28.4% of patients carried HRRm. Among the common HRR mutation types (BRCA2, ATM, CDK12), all showed survival benefits favoring olaparib, with BRCA2 mutation patients having an rPFS and OS HR of only 0.20; ATM mutation patients had an rPFS and OS HR of 0.55 and 0.79, respectively; CDK12 mutation patients had an rPFS and OS HR of 0.51 and 0.57, respectively.
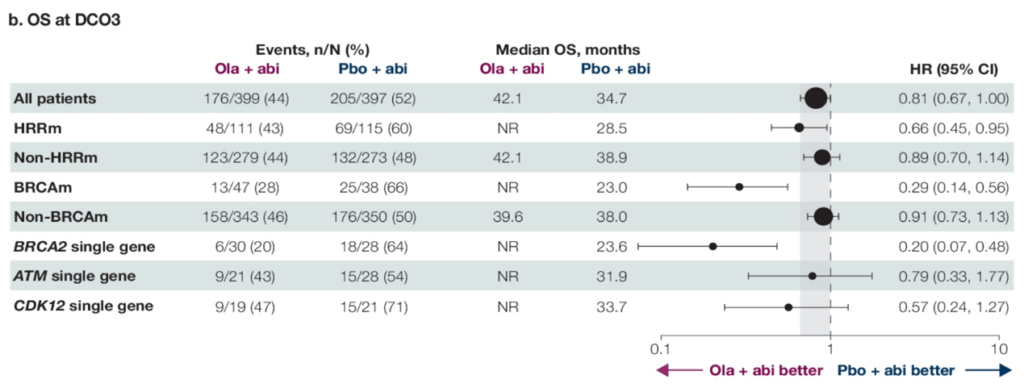
△ OS Results for HRRm Subgroup Patients in the PROpel Study Reported at ASCO-GU 2024
Given the exceptional efficacy of PARP inhibitors, we should enhance genetic testing and screening for advanced prostate cancer patients to precisely identify those who may benefit from targeted treatment with PARP inhibitors like olaparib. Consequently, the 2023 CSCO guidelines for prostate cancer recommend genetic testing for homologous recombination repair-related genes (including BRCA1/2) for patients with metastatic prostate cancer (including mHSPC and mCRPC) as a level I recommendation; mismatch repair and other DNA repair-related gene testing as a level II recommendation; and testing for other genes related to the treatment and prognosis of prostate cancer as a level III recommendation.
Olaparib’s indication for treating mCRPC has been approved in China and has also been included in the national health insurance, making it the only PARP inhibitor approved for prostate cancer indication and covered by the national health insurance in the country. Hematologic adverse reactions may be one of the main issues with the use of PARP inhibitors. Internationally, studies like PROpel, TALAPRO-2, and MAGNITUDE have reported on first-line treatments for mCRPC with PARPi. Overall, olaparib combined with NHA has fewer blood side effects, with a grade ≥3 anemia incidence rate of 16.1%, compared to 46.5% for talazoparib plus NHA and 29.7% for niraparib plus NHA (not head-to-head studies).
In clinical practice, urologists need to accurately recognize related adverse reactions, actively communicate with patients, conduct regular follow-ups for patients treated with olaparib, and proactively manage any adverse reactions that occur, to improve treatment adherence and thereby enhance patient outcomes.


