Editor's Note: Following the breakthrough of the PROfound study, which marked the beginning of precision treatment for prostate cancer, the PROpel study has further extended the application of PARP inhibitors (PARPi) to the frontline treatment of metastatic castration-resistant prostate cancer (mCRPC). The recently 2024 ASCO-GU Symposium published the PROpel study subgroup data for patients with homologous recombination repair (HRR) gene mutations. "Oncology Frontier" invited Dr. Pengfei Shen from West China Hospital, Sichuan University, to interpret the PROpel study and share his views on advancing genetic testing to guide precision treatments for advanced prostate cancer.
Oncology Frontier : This ASCO-GU Symposium published several latest researches related to PARP inhibitors. Among them, the PROpel study revealed the efficacy of olaparib in combination with abiraterone compared to placebo with abiraterone in mCRPC patients with HRR mutations. Could you share with us the latest research data?
Dr. Pengfei Shen: The PROpel study is a prospective, multicenter, phase III randomized controlled trial designed to evaluate the efficacy and safety of olaparib combined with abiraterone as a first-line treatment for mCRPC. Patients were enrolled without HRR gene testing selection and randomly assigned in a 1:1 ratio to the olaparib + abiraterone group or the placebo + abiraterone group. The primary endpoint was radiographic progression-free survival (rPFS), with secondary endpoints including time to first subsequent therapy (TFST), time to second disease progression (PFS2), overall survival (OS). Results presented at the 2022 ASCO-GU Symposium showed that the study met its primary endpoint: in the overall population, the median rPFS was significantly longer in the olaparib group compared to the placebo group (24.8 vs 16.6 months, HR 0.66, P<0.0001). Among key secondary endpoints: there was a trend towards benefit in OS in the overall population for the olaparib group (42.1 vs 34.7 months, HR 0.81, P=0.0544).
The detailed data for the HRRm population was reported at this ASCO-GU Symposium: overall, 28.4% of patients carried HRRm, with consistent benefit in rPFS observed across non-HRR mutations and different HRR mutation genes. OS showed survival advantages favoring olaparib in common HRR mutation types (BRCA2, ATM, CDK12), with BRCA2 mutation patients having rPFS and OS HRs of only 0.20; ATM mutation patients had rPFS and OS HRs of 0.55 and 0.79, respectively; CDK12 mutation patients had rPFS and OS HRs of 0.51 and 0.57, respectively.
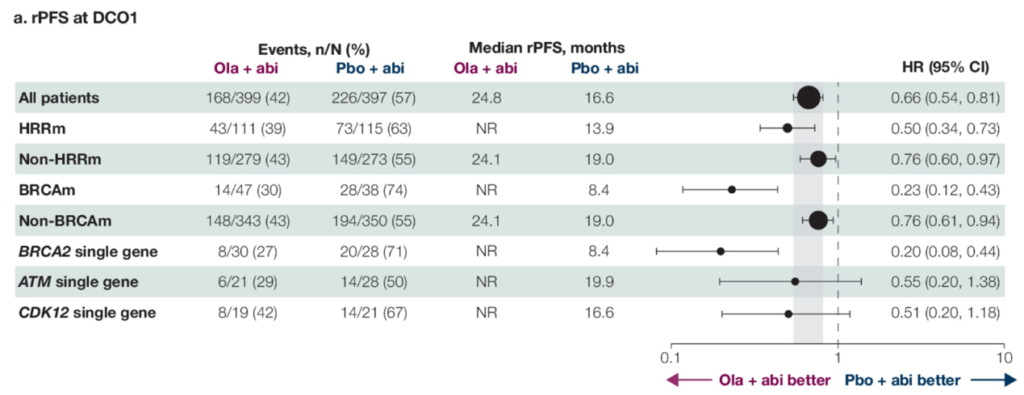
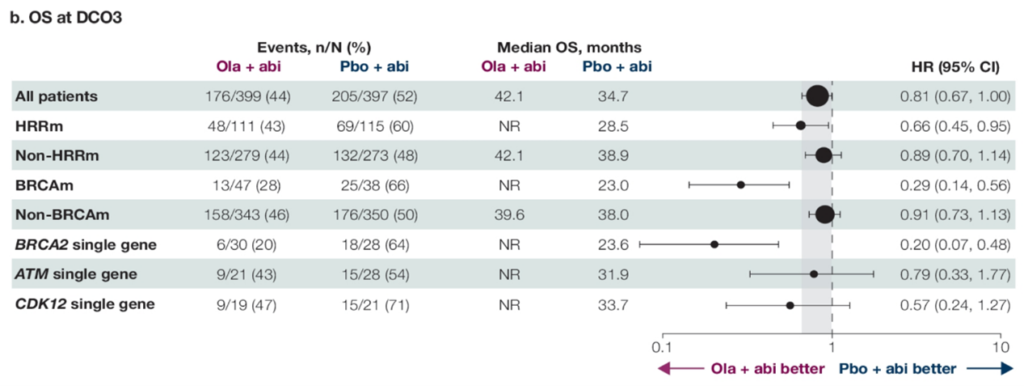
△PROpel Study’s rPFS and OS for the Entire Cohort, Non-HRRm, and HRRm Patients (Including Various HRR Gene Mutations)
The results of the PROpel study demonstrated a notable OS benefit in the HRRm patient group, offering significant insights for clinical practice: First, the importance of genetic testing. The series of studies on olaparib in the mCRPC domain emphasize the importance of comprehensive genetic testing before making treatment decisions. Identifying patients with HRR gene mutations allows doctors to more precisely select the patient group most likely to benefit, implementing personalized treatment decisions. Second, the advancement of precision medicine. The significant therapeutic response of the HRRm patient group to PARP inhibitors highlights the potential of precision medicine in prostate cancer treatment. These studies underscore the concept of guiding treatment choices based on the specific genetic characteristics of the patient in prostate cancer treatment decisions. Third, the timely change in treatment paradigms and strategies. For castration-resistant prostate cancer patients, especially those carrying HRRm, considering PARP inhibitors as a first-line treatment option should be contemplated. This could alter existing treatment strategies, introducing gene-targeted therapy earlier in the treatment process.
Oncology Frontier : Research on BRCAm/HRRm guiding PARPi treatment for mCRPC has accumulated a wealth of evidence-based medical evidence. However, there are many types of HRR-related mutations. What requirements or insights do you think this presents for clinical genetic testing?
Dr. Pengfei Shen: There are many types of HRR-related gene mutations, and besides HRR mutations, many other gene mutations are also expected to impact clinical practice and guide diagnostic and treatment decisions. Therefore, clinical genetic testing needs to adapt and improve in the following areas.
First, broad panel testing may help to discover more meaningful gene mutations. There are many types of HRR mutations, with BRCA1/2 being the most common type, accounting for about 9.7% (with incidence rates up to 12.1% in East Asian populations). In addition, there are other gene mutations such as ATM, CDK12, CHEK, etc. From the PROpel study’s HRRm-related results reported at this ASCO-GU, besides BRCA1/2, the combination treatment of olaparib and abiraterone for patients with mutations in ATM, CDK12, CHEK2, etc., also showed good survival benefits. Therefore, in clinical testing, it is advisable to cover as many HRR-related mutation gene tests as possible, and a broad panel can help identify more HRRm patients, where economically feasible.
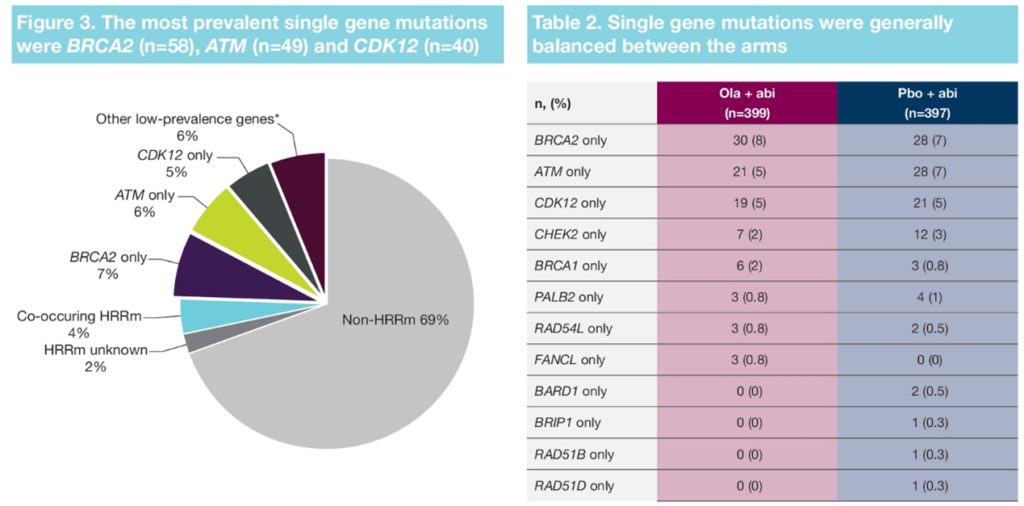
△Distribution of HRR Mutation Genes in Patients Enrolled in the PROpel Study
Moreover, the choice of sample for genetic testing is particularly important for patients with advanced prostate cancer. For BRCA/HRR mutation testing, tissue sample testing remains the gold standard. However, in actual clinical practice, the choice of sample depends on several factors, including the patient’s clinical condition (whether they can undergo a biopsy), the availability of the sample (insufficient archived tissue or outdated samples), the biological characteristics of the tumor, and the type of genetic information needed. Using a combination of these sample types can provide a more comprehensive set of genetic information, helping doctors to formulate more precise treatment strategies. For patients with advanced prostate cancer, obtaining tissue samples that are accessible within 2 years is challenging, and the failure rate of genetic testing reports increases with the age of the stored tissue samples. When tissue samples are not readily available, ctDNA is an effective alternative. The CSCO guidelines for the diagnosis and treatment of prostate cancer suggest that ctDNA has a consistency rate of over 80% and can serve as an effective substitute for tissue samples.
Third, the application of ctDNA testing is becoming more widespread, with promising prospects for the future. A study reported at this ASCO-GU conference showed that in patients with mCRPC with liver metastases, ctDNA testing is more likely to detect mutations in genes such as AR, BRAF, BRCA2, CDK6, EGFR, FGFR1, MYC, and PIK3CA. Currently, besides PARP inhibitors, targeted therapy drugs for the PI3K-AKT-mTOR pathway are under development, particularly the AKT inhibitor Capivasertib, which has entered phase III clinical trials (CAPItello-281 study). Therefore, using a broad panel ctDNA test will help to identify more targets with potential therapeutic value.
Reference :
- Neal D. Shore,et al.Efficacy of olaparib (O) plus abiraterone (A) versus placebo (P) plus A in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with single homologous recombination repair gene mutations (HRRm) in the PROpel trial.ASCO-GU 2024,Abstract #165
- Minqi Huang,et al.Evaluation of ctDNA in patients with mCRPC with liver metastases.ASCO-GU 2024,Abstract #212
- Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437-1447.
- Shi, Zhen et al. Biomarker analysis of the phase III IPATential150 trial of first-line ipatasertib (Ipat) plus abiraterone (Abi) in metastatic castration-resistant prostate cancer (mCRPC).Journal of Clinical Oncology 38, no. 6_suppl (2020): 182–82


