
The European Society for Medical Oncology Congress 2024(ESMO 2024) opened on September 13, in Barcelona,Spain. Lung cancer remains one of the key areas of focus,with several groundbreaking studies presented at the conference. Oncology Frontier invited Dr.Yuankai Shi, principal investigator of the INSPIRE study from the Cancer Hospital Chinese Academy of Medical Sciences,to share the latest data from this year's ESMO conference and discuss the clinical value of the study in treating anaplastic lymphoma kinase(ALK)-positive advanced non-small cell lung cancer(NSCLC).Q1: Congratulations on the INSPIRE study once again presenting significant findings once again at an international academic conference. Could you provide an overview of the key data updates from the INSPIRE study presented at the ESMO Congress 2024?
Dr.Yuankai Shi: ALK fusion is considered the “diamond target”in the treatment of NSCLC; and providing timely and effective ALK-TKI targeted therapy can significantly prolong survival for patients with ALK-positive advanced NSCLC.At the 2023 World Conference on Lung Cancer (WCLC),the INSPIRE study—a head-to-head Phase Il trial comparing the next- generation ALK-TKI Iruplinalkib against Crizotinib as first-line therapy for ALK-positive advanced NSCLC—reported interim results for the first time.The data showed that the median progression-free survival (PFS)for the Iruplinalkib group was 27.7 months compared to 14.6 months for the Crizotinibgroup,with a hazard ratio (HR)of 0.34(P<0.0001) [1].In addition to the significant median PFS,Iruplinalkib demonstrated notable intracranial efficacy: among patients with measurable baseline intracranial metastases,the intracranial objective response rate (ORR) was 90.9%for Iruplinalkib versus 60.0% for Crizotinib. For patients with central nervous system (CNS) metastases at baseline,the intracranial ORR was 57.9% in the Iruplinalkib group compared to 25.6% in the Crizotinib group.
At this year’s ESMO conference,the INSPIRE study was featured again(Poster 1278P).
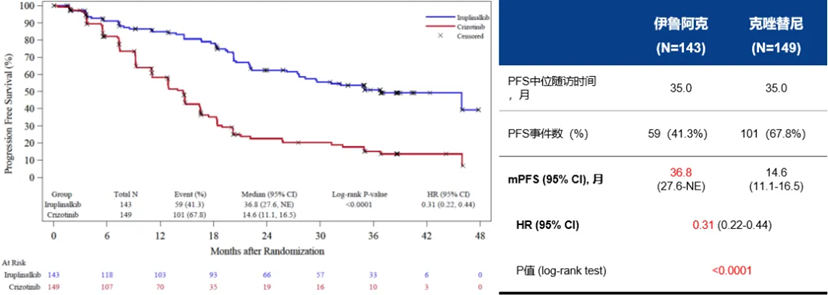
Figure 1. Updated PFS (IRC-assessed) results from the INSPIRE study after extended follow-up
The cutoff date for this updated analysis was October 25,2023,with a median follow-up time of 35.0 months for both the Iruplinalkib and Crizotinib groups,as assessed by the independent review committee (IRC).The median PFS was 36.8 months for Iruplinalkib versus 14.6 months for Crizotinib,with an HR of 0.31(P<0.0001).For patients with CNS metastases at baseline,the IRC-assessed median PFS was 26.3 months for Iruplinalkib compared to 11.0 months for Crizotinib(HR=0.21,P<0.0001).For patients without CNS metastases at baseline,the IRC-assessed median PFS was 45.9 months for Iruplinalkib and 16.4 months for Crizotinib(HR=0.33,P<0.0001).Additionally,the 36-month overall survival (OS)rate was 81.3% for the Iruplinalkib group compared to 75.8% for the Crizotinib group.
In terms of safety,the findings were consistent with previous results,and no new safety concerns were observed.The incidence of grade 3 or higher treatment-related adverse events (TRAEs)was 53.1%in the Iruplinalkib group and 51.0%in the Crizotinib group.
In summary,the study demonstrated that Iruplinalkib consistently improves PFS in treatment-naïve ALK-positive advanced NSCLC patients compared to Crizotinib. It also showed that Iruplinalkib’s strong intracranial efficacy reduced the risk of disease progression or death by 79%in patients with baseline brain metastases,making it an optimal first-line treatment option for ALK-positive advanced NSCLC.
Q2: From your perspective,what is the clinical value of the updated INSPIRE study data,and how might it benefit the treatment of Chinese patients with ALK-positive locally advanced or metastatic NSCLC?
Dr.Yuankai Shi: Currently,eight ALK-TKIs have been approved in China,significantly improving the survival outcomes for Chinese patients with ALK-positive advanced NSCLC and supporting the long-term management of this condition.Among these ALK-TKls,the Chinese-developed innovative drug Iruplinalkib,which has demonstrated a favourable balance between efficacy and safety,has become an important first-line treatment option for ALK-positive advanced NSCLC patients.
The updated INSPIRE study results showed that the median PFS for Iruplinalkib extended to 36.8 months,reducing the risk of disease progression or death by 69% compared to Crizotinib[2].In subgroup analyses,Iruplinalkib also demonstrated significant intracranial efficacy in patients with baseline CNS metastases.These updated data suggest that with extended follow-up,patients continue to derive substantial benefits,as reflected in the prolonged median PFS.
Based on the excellent efficacy and safety profile of Iruplinalkib,the drug has been included in multiple guidelines,recommendations,and expert consensus documents, including the China Clinical Practice Guideline for Stage IV Primary Lung Cancer (2024 Edition) ,China Expert Recommendations on Anaplastic Lymphoma Kinase-Tyrosine Kinase Inhibitors Treatment for Advanced Non-Small Cell Lung Cancer (2024 Edition), Guidelines of Chinese Society of Clinical Oncology (CSCO) Non-Small Cell Lung Cancer (2024 Edition) and the Chinese Medical Association Guidelines for ClinicalDiagnosis and Treatmentof Lung Cancer (2024 Edition)[3-6]. This will further facilitate its clinical use following approval and is expected to benefit more Chinese patients with ALK-positive advanced NSCLC.
In terms of safety,the 2022 White Paper on the Survival Status of ALK-Positive NSCLC Patients pointed out that adverse events such as edema,diarrhea,vomiting,and constipation from targeted therapies can negatively affect the patient’s quality of life[8].In a large-scale analysis of 409 Chinese patients, Iruplinalkib demonstrated a lower incidence of muscle pain, edema, and constipation. Additionally, the risk of QTc interval prolongation and hyperglycemia was significantly reduced, offering patients an improved treatment experience and enhanced quality of life.
All patients in the INSPIRE study were from China,making it a large-scale clinical study specifically focused on Chinese patients.The clinical dosing,efficacy,and safety data align closely with the clinical characteristics and needs of Chinese patients,offering valuable guidance for clinical treatment decisions in this population .
References
1. Y. Shi, J. Chen, R. Yang, et al. A Randomized, Phase III Study of Iruplinalkib (WX-0593) vs Crizotinib in ALK TKI-Naïve, Locally Advanced or Metastatic ALK-Positive Non-Small Cell Lung Cancer (INSPIRE). WCLC 2023. OA03.05.
2. Y. Shi, J. Chen, R. Yang, et al. Update of the INSPIRE study: Iruplinalkib versus Crizotinib in ALK TKI-naïve locally advanced or metastatic ALK+ non-small cell lung cancer (NSCLC). 2024 ESMO, 1278P.
3.《IV期原发性肺癌中国治疗指南(2024版)》. 中华肿瘤杂志[J]. 2024,46(7): 595-.-636.
4.《ALK抑制剂治疗晚期非小细胞肺癌中国专家建议(2024版)》. 中华医学杂志[J]. 2024, 104(7) : 473-485.
5.《CSCO非小细胞肺癌诊疗指南2024》.
6.《中华医学会肺癌临床诊疗指南(2024版)》. 中华肿瘤杂志[J]. 2024,46(9): 805-843.
7.Liu XL, Zhang L, Wan HW, et al. Discovery and preclinical evaluations of WX-0593, a novel ALK inhibitor targeting Crizotinib-resistant mutations. Bioorganic & Medicinal Chemistry Letters. 2022 Jun 15: 66: 128730.
8.《2022非小细胞肺癌ALK阳性患者生存现状调研白皮书》
Yuankai Shi, MD, PhD
l Professor of Oncology and Chief Physician, Department of Medical Oncology
l Doctoral Supervisor, Cancer Hospital Chinese Academy of Medical Sciences
l Professor, Peking Union Medical College (long-term appointment)
l Founding President, First and Second Terms, Tumor Physician Branch, Chinese Medical Doctor Association
l Chairman, Third and Fourth Terms, Tumor Clinical Chemotherapy Professional Committee, Chinese Anti-Cancer Association
l Chairman, Fifth Term, Lymphoma Professional Committee, Chinese Anti-Cancer Association
l Founding Chairman, First and Second Terms, Anti-tumor Drug Professional Committee, Chinese Pharmaceutical Association
l Founding Chairman, Department of Medical Oncology, Chinese Medical Association
l Member, Drug Evaluation Expert Advisory Committee, National Medical Products Administration (NMPA)
l Member, First and Second Technology Innovation Advisory Committee, Shanghai Stock Exchange
l President, Eighth Term, China Cancer Foundation
l Founding President, Xiongan New Area Medical Association


