Editor’s Note: Triple-negative breast cancer (TNBC) is a highly heterogeneous subtype of breast cancer that is more aggressive and associated with a higher risk of early recurrence, metastasis, and death compared to other subtypes. The team led by Professors Zhimin Shao and Yizhou Jiang from the Breast Surgery Department at Fudan University Shanghai Cancer Center used a previously established mRNA-lncRNA predictive model to determine the effectiveness and safety of intensive versus standard chemotherapy in high-risk TNBC patients. The results were selected for the 2024 ASCO conference (Abstract No. 525). Professor Zhonghua Wang, representing the team at the conference, provided an introduction to the findings for "Oncology Frontier"Effect of Intensive Chemotherapy vs Standard Chemotherapy on Disease-Free Survival Among Patients With High-Risk Operable Triple-Negative Breast Cancer Based on An Integrated mRNA–lncRNA Signature: The Randomized BCTOP-T-A01 Clinical Trial
Background
Triple-negative breast cancer (TNBC) is a highly heterogeneous subtype of breast cancer. Compared to other breast cancer subtypes (hormone receptor-positive and HER2-positive), TNBC exhibits more aggressive biological behavior and is associated with higher risks of early recurrence, metastasis, and death [1,2]. Adjuvant chemotherapy based on anthracyclines or taxanes is the standard treatment for early TNBC, but about 20% to 40% of patients experience disease recurrence. Therefore, more effective strategies are urgently needed to optimize adjuvant therapy for TNBC. Several multigene models have been used to predict prognosis and guide adjuvant therapy in other breast cancer subtypes. However, there is still a lack of prospectively validated TNBC-specific predictive models.
The research team previously developed a prognostic model for TNBC based on mRNA-lncRNA expression profiles, categorizing TNBC patients into high-risk and low-risk recurrence groups [6]. The model’s high-risk and low-risk patients showed significant survival differences. Further interaction analysis indicated that high-risk patients identified by the predictive model had limited benefits from taxane chemotherapy. Based on this preliminary research, the researchers conducted the BCTOP-T-A01 phase III clinical trial to compare the efficacy and safety of intensive (TEC4-GP4) versus standard (EC4-T4) adjuvant chemotherapy in high-risk TNBC patients. Additionally, the study aimed to prospectively validate the predictive sensitivity and specificity of the model.
Methods
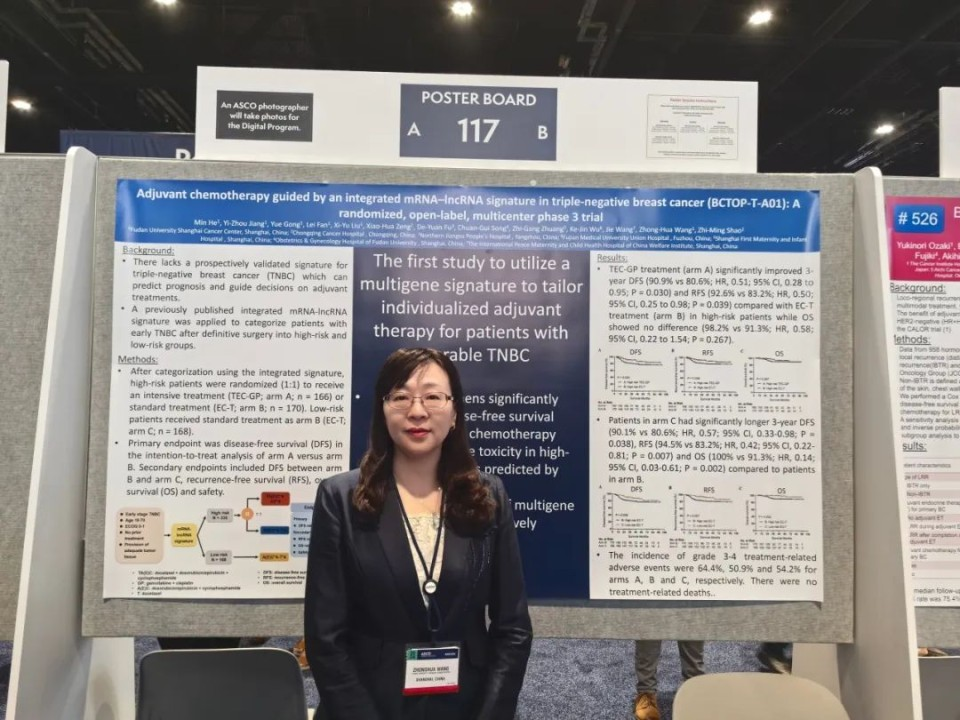
BCTOP-T-A01 is a randomized, open-label, phase III clinical trial that enrolled patients aged 18-70 with unilateral invasive breast cancer who had not received neoadjuvant therapy and were histologically and immunohistochemically confirmed as early-stage (M0) triple-negative breast cancer. Patients had either positive or negative lymph nodes with a tumor size >1 cm. Based on the results of surgical specimen testing by the central laboratory, patients were categorized into high-risk and low-risk groups using the previously established mRNA-lncRNA predictive model. High-risk patients were randomly assigned 1:1 to receive intensive or standard adjuvant chemotherapy. The intensive group received four cycles of docetaxel, epirubicin, and cyclophosphamide followed by four cycles of gemcitabine and cisplatin (TEC4-GP4), while the control group received four cycles of epirubicin and cyclophosphamide followed by four cycles of docetaxel (EC4-T4). Low-risk patients also received the standard regimen (EC4-T4). From January 2016 to July 2023, 504 patients were enrolled, with 336 classified as high-risk by the mRNA-lncRNA model and 168 as low-risk. The primary endpoint was disease-free survival (DFS) in high-risk patients between the intensive and standard treatment groups. Secondary endpoints included DFS in high-risk vs. low-risk patients receiving the same standard treatment, recurrence-free survival (RFS), overall survival (OS), and safety.
Results
After a median follow-up of 45 months, results indicated that the 3-year DFS rate in the high-risk intensive treatment group was 90.9%, significantly better than the 80.6% in the standard treatment group (HR, 0.51; 95% CI: 0.28–0.95; P=0.030). Additionally, the 3-year RFS rate was significantly higher in the intensive group compared to the standard group (92.6% vs. 83.2%; HR, 0.50; 95% CI: 0.25–0.98; P=0.039). As of the data analysis, the 3-year OS rates were 98.2% and 91.3% for the intensive and standard groups, respectively (P=0.267); longer follow-up is needed to assess the impact of intensive chemotherapy on OS.
Compared to high-risk patients receiving the same standard chemotherapy, low-risk patients had significantly better 3-year DFS (90.1% vs. 80.6%; HR, 0.57; 95% CI: 0.33–0.98; P=0.038), RFS (94.5% vs. 83.2%; HR, 0.42; 95% CI: 0.22–0.81; P=0.007), and OS (100% vs. 91.3%; HR, 0.14; 95% CI: 0.03–0.61; P=0.002).
The incidence of grade 3-4 treatment-related adverse events was 64.4%, 50.9%, and 54.2% in the high-risk intensive treatment group, high-risk standard treatment group, and low-risk group, respectively. No treatment-related deaths were observed in the entire cohort.
Conclusion
The mRNA-lncRNA model accurately predicts postoperative recurrence risk in operable TNBC patients. The addition of gemcitabine and cisplatin to the anthracycline/taxane-based regimen significantly improves DFS in high-risk patients identified by the model. Although the intensive treatment regimen increases the incidence of adverse events compared to the control group, the toxicity is generally manageable.


