Editor's Note: The 18th St. Gallen International Breast Cancer Conference (SGBCC 2023) commenced in Vienna, the "City of Music," on March 15, 2023. During the "SGBCC Academy" session of the conference, Professor Jiang Zefei from the Oncology Department of the Chinese People's Liberation Army General Hospital delivered a presentation titled "Who can benefit from adjuvant therapy with CDK4/6 inhibitors?" which received unanimous praise. Subsequently, "Tumor Outlook" conducted an exclusive interview with Professor Jiang Zefei, discussing the current landscape of CDK4/6 inhibitors in breast cancer systemic therapy and their applicability to different patient populations.oncology frontier : The development of CDK4/6 inhibitors has brought more possibilities for the treatment of hormone receptor-positive breast cancer. Could you please discuss the role and status of CDK4/6 inhibitors in systemic therapy for breast cancer?
Professor Jiang Zefei: When it comes to targeted therapy for breast cancer patients, we are familiar with drugs targeting HER2, followed by those targeting hormone therapy. I remember about seven or eight years ago, when we were discussing at some international conferences, we were just beginning to hear about CDK4/6 inhibitors. Now, CDK4/6 inhibitors are being used more and more in clinical practice. In the previous St. Gallen conference, we discussed the feasibility of advancing CDK4/6 inhibitors from advanced to early stage. Based on the results of the monarchE clinical study, more and more scholars are accepting the concept that “some HR+ breast cancer patients at high risk of recurrence should receive combination therapy with CDK4/6 inhibitors in adjuvant endocrine therapy.” The two-year follow-up results of the monarchE study further confirm the clinical status and proper role of abemaciclib.
oncology frontier: In the SGBCC Academy of this conference, you presented “Who can benefit from adjuvant therapy with CDK4/6 inhibitors?” Could you review the content of your presentation and discuss which early-stage breast cancer patients can benefit from CDK4/6 inhibitor therapy?
Professor Jiang Zefei: At the invitation of the conference, I presented on “Who can truly benefit from adjuvant therapy with CDK4/6 inhibitors.” I first reviewed the results of the monarchE clinical study and analyzed the population who truly benefit from abemaciclib treatment and the magnitude of their benefit. Patients with ≥4 positive axillary lymph nodes (ALN) will experience absolute benefit. Additionally, I analyzed whether Ki-67 is an independent factor for patient benefit in the group with “1-3 positive ALN and G3 or T≥5cm, Ki-67≥20%.” I believe that as long as patients have relevant high-risk factors, they can benefit from abemaciclib treatment.

Source: SGBCC Official Website
In fact, regulatory agencies have been gradually changing their approvals for relevant indications in line with clinical data. Recently, the FDA approved the expansion of indications for abemaciclib in combination with endocrine therapy, extending its use to adult patients with HR+/HER2-, lymph node-positive, early-stage breast cancer at high risk of recurrence for adjuvant treatment. The expanded indications for adjuvant treatment have removed the requirement for patient Ki-67 scoring. The 2023 St. Gallen Conference and the Consensus Voting Session of the North-South Summit also discussed relevant content. It is believed that the China Drug Evaluation (CDE) will also follow the results of these discussions and scientific research.
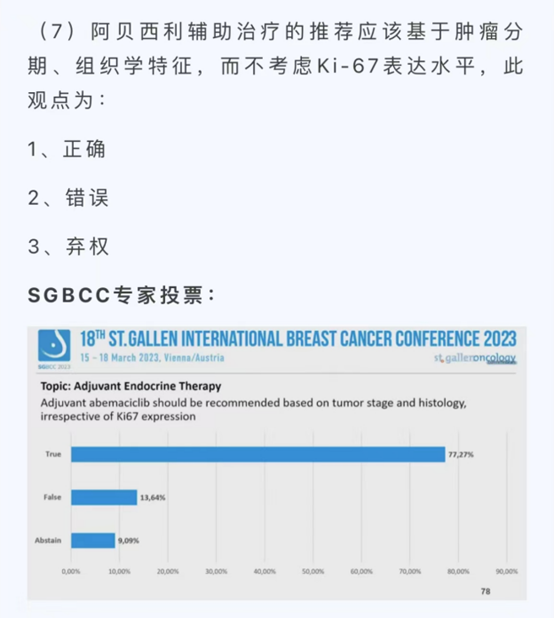

2023 St. Gallen Conference Consensus Voting
In my opinion, the approval of indications for a product should be based on the characteristics of the patient population enrolled in relevant clinical studies. Therefore, in last year’s CSCO Breast Cancer (BC) Guidelines, we clearly defined the recommended population for the use of abemaciclib in adjuvant endocrine therapy for HR+ postmenopausal breast cancer patients as “≥4 positive axillary lymph nodes (ALN), 1-3 positive ALN with G3 or T≥5cm, Ki-67≥20%.” It is also worth noting that some patients with poor response to neoadjuvant chemotherapy were included in the monarchE study. Just as we use T-DM1 for intensified treatment in HER2-positive breast cancer patients who do not achieve pathological complete response (non-pCR) with dual HER2-targeted therapy, similarly, for HR+ breast cancer patients with poor response to neoadjuvant chemotherapy, intensified treatment can be considered postoperatively, with the option of endocrine therapy combined with abemaciclib. The above is the main content I presented at the SGBCC Academy.
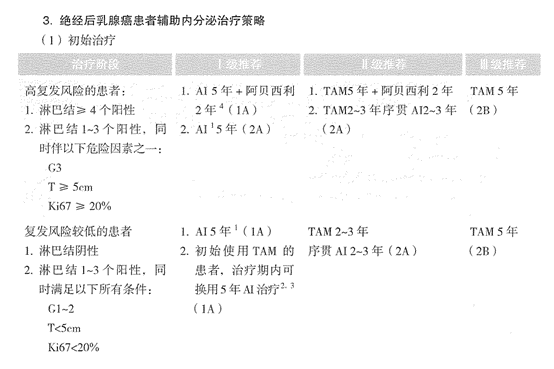
△ CSCO BC Guidelines (2022) Adjuvant Endocrine Therapy Strategies for Postmenopausal Breast Cancer Patients
oncology frontier : With the current surge of CDK4/6 inhibitors, could you share how clinicians should strategize and choose the appropriate CDK4/6 inhibitor for patients in clinical practice?
Professor Jiang Zefei: In clinical practice, patients can be divided into two stages. For early-stage breast cancer, currently, only abemaciclib (based on the results of the monarchE study) has obtained approval from regulatory authorities and recognition in guidelines both domestically and internationally. For patients with advanced breast cancer, there are currently both domestically produced original products and imported products of CDK4/6 inhibitors available clinically. We should make rational choices based on the patient’s actual situation, the population included in each clinical study, different product characteristics, and medical insurance coverage.
What is worth looking forward to is that in the 2023 CSCO BC Guidelines, which will be announced on April 7th, we have positioned the current status of different CDK4/6 inhibitors based on the patient’s treatment stratification. The reason for dividing into different levels is to provide reasonable recommendations based on the evidence-based medicine evidence, safety, and medical insurance reimbursement status of the drug.
oncology frontier : Based on studies like monarchE and MONARCH plus, abemaciclib has become the CDK4/6 inhibitor that comprehensively covers hormone receptor-positive breast cancer from early to advanced stages in China. What are your expectations for its future application and research exploration in China?
Professor Jiang Zefei: Our center was fortunate to participate in the monarchE study, and as the PI, I also led the international multicenter MONARCH plus study. Participation in multiple studies has allowed us to accumulate valuable experience and appreciate the excellent treatment effect of abemaciclib. As abemaciclib transitions from advanced to early stages, we also hope to find more suitable populations, safer management strategies, and more combination therapy options, so that it can benefit more breast cancer patients. We truly hope it can help advanced patients achieve longer survival and provide more opportunities for cure for early-stage patients.


