Editor's Note: The annual American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO-GU 2024) was held in San Francisco, USA, from January 25 to 27, 2024. The conference presented updates on the PROpel study regarding patients with homologous recombination repair (HRR) gene mutations, and featured oral presentations on the BRCAAway study, which explored the use of olaparib alone or in combination with abiraterone as first-line treatment for metastatic castration-resistant prostate cancer (mCRPC). These studies highlight the importance of precision therapy strategies for prostate cancer, emphasizing the need for timely genetic testing for advanced prostate cancer patients. This enables patients with BRCA1/2 and other HRR mutations to benefit from PARP inhibitors like olaparib. Dr. Xuefeng Qiu from the Drum Tower Hospital, Medical School of Nanjing University, shared insights into these research advancements and the clinical application of olaparib in an interview with Oncology Frontier.
Oncology Frontier: At this year’s conference, research and application of PARP inhibitors, particularly olaparib, in advanced prostate cancer have emerged as hot topics. Could you discuss the new data and explorations related to olaparib presented at ASCO-GU?
Dr. Xuefeng Qiu: Olaparib, as the first PARP inhibitor to enter the field of prostate cancer, has ushered in the era of precision therapy for the disease. Previous findings from the PROfound study have shown that for mCRPC patients with BRCA mutations who failed next-generation hormonal agent (NHA) treatments, olaparib significantly improved progression-free survival (PFS) (9.8 vs. 3.0 months, HR 0.22) and overall survival (OS) (20.1 vs. 14.4 months, HR 0.63). Consequently, guidelines recommend olaparib for mCRPC patients who fail NHA treatments and are found to have BRCA1/2 mutations through genetic testing.
The PROpel study reported in 2022 further explored the efficacy and safety of using olaparib in combination with abiraterone and ADT versus placebo with abiraterone and ADT in the first-line treatment of mCRPC, in patients not selected through genetic testing. The initial results were very encouraging, showing a significant extension in radiographic progression-free survival (rPFS) in the olaparib group compared to the placebo group (24.8 vs. 16.6 months, HR 0.66, P<0.0001). Unfortunately, the overall survival (OS) for the entire population did not meet the threshold for statistical significance (42.1 vs. 34.7 months, HR 0.81, P=0.0544). However, subsequent subgroup analyses revealed better OS benefits for patients with BRCA mutations (HR 0.29) or HRR mutations (HR 0.66). This year’s ASCO-GU further reported on the efficacy in different HRR mutation types, showing that patients with common HRR mutations like BRCA2 (HR 0.20), ATM (HR 0.79), CDK12 (HR 0.57) also benefited in terms of OS. Currently, international guidelines such as NCCN also recommend genetic testing for mCRPC patients, suggesting the use of olaparib in combination with abiraterone as a first-line treatment for patients with BRCA1/2 or other HRR gene mutations.
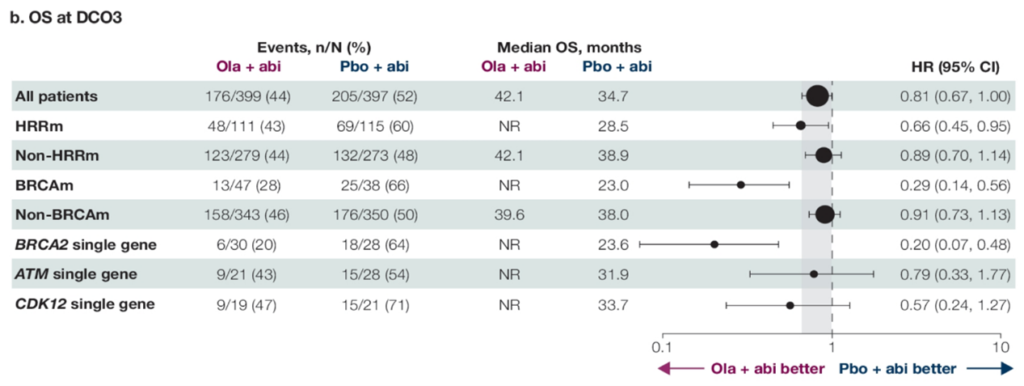
The PROpel study provided insights into the overall survival (OS) for the entire cohort, patients without HRR mutations (non-HRRm), and those with HRR mutations (HRRm), including various HRR gene mutations, in the context of combining PARP inhibitors with novel endocrine therapy for mCRPC treatment. This year’s ASCO-GU presented numerous studies expanding the first-line treatment options for mCRPC patients. Both the PROfound and PROpel studies have confirmed the benefits of olaparib in the second-line and first-line treatment of mCRPC, respectively. Therefore, a key question addressed at the conference was whether olaparib should be used in the first-line or second-line setting. The BRCAAway study presented at this conference attempted to answer this question by enrolling mCRPC patients with HRR mutations for first-line treatment. Cohorts 1-3 consisted of patients with BRCA1/2 or ATM mutations, receiving either abiraterone alone, olaparib alone, or a combination of olaparib and abiraterone. The results indicated a trend of benefit across all three groups, with the combination of olaparib and abiraterone showing the most significant benefit. The median progression-free survival (PFS) reached 39 months for the combination treatment, compared to 8.5 months for abiraterone alone and 14 months for olaparib alone.
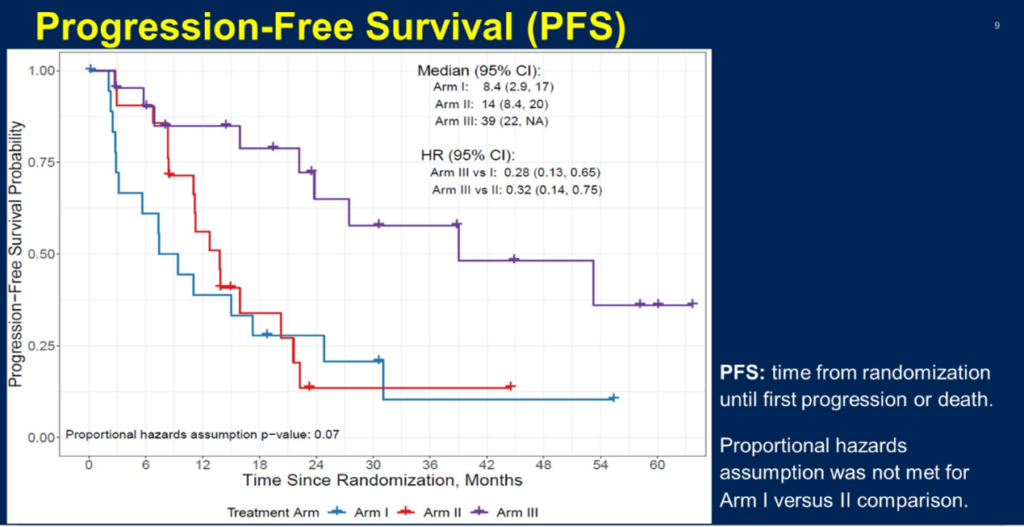
The BRCAAway study’s cohorts 1-3 PFS, alongside the PROfound and PROpel studies, indicate that for patients carrying HRR mutations such as BRCA1/2, the combination of olaparib with abiraterone offers the most significant treatment benefit. These novel data highlight the value of PARP inhibitors in the management of advanced prostate cancer. As research continues to evolve and delve deeper, we expect the application range of PARP inhibitors like olaparib in mCRPC to broaden, benefiting an even larger group of patients. However, genetic testing remains a crucial prerequisite for selecting PARP inhibitor treatment.
Oncology Frontier: Olaparib has shown considerable benefits in the precision diagnosis and treatment of advanced prostate cancer. How do you think clinical management should be conducted when using olaparib for treating mCRPC to optimal patient management to improve quality of care?
Dr. Xuefeng Qiu: This question arises as olaparib enters clinical practice for mCRPC and addresses a very practical issue. Currently, the indication for olaparib treatment of mCRPC has been approved in China and has successfully been included in the national medical insurance system, making it the only PARP inhibitor approved for prostate cancer in China and widely used in clinical practice. Managing its side effects well is crucial in clinical settings. On the other hand, the current domestic indication for olaparib is mainly based on the PROfound study, targeting mCRPC patients with BRCA mutations, hence genetic testing is key to guiding treatment choices.
Studies like PROpel and BRCAAway suggest we need to change our treatment strategies, advancing the timing for olaparib treatment and genetic testing. A retrospective analysis involving 661 mCRPC patients showed that the proportion of patients experiencing clinical progression increased with the number of treatment lines: 43% in the first line and 68% in the third line. Therefore, to extend the survival of prostate cancer patients, especially those with mCRPC, it’s vital to recommend genetic testing early, enabling patients with HRR mutations like BRCA1/2 to receive timely treatment with PARP inhibitors such as olaparib, maximizing their treatment benefits. In managing adverse reactions, PARP inhibitors and novel hormonal agents (NHA) have their unique side effects, especially when used in combination, where cumulative toxicities may occur. The common adverse events for olaparib mainly include hematologic toxicities like anemia, while NHAs are primarily associated with metabolic or cardiovascular diseases like osteoporosis and hypertension. Ensuring patient safety to prolong treatment duration is essential; discontinuing treatment due to poor management would be regrettable.
Overall, mCRPC has entered the era of precision medicine, where genetic testing can reveal more potential treatment options for patients. The future will likely focus on multimodal diagnostic and treatment approaches, including various monotherapies and combination therapies. Based on the PROpel and BRCAAway studies, the combination of a PARP inhibitor with an NHA appears to be the best approach for patients carrying HRR mutations like BRCA1/2. In this multimodal treatment trend, establishing a multidisciplinary team (MDT) collaboration platform is necessary, allowing doctors from different specialties to participate in patient diagnosis, treatment decisions, and management, including understanding the mechanisms and potential side effects of different drugs to provide scientific medication guidance. Urologists need to accurately recognize treatment-related adverse effects and manage patients receiving olaparib treatment with proper follow-up and adverse reaction management, thereby improving patient compliance and enhancing treatment outcomes.
Reference :
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094-1108.
- NCCN Clinical Practice Guidelines in Oncology:Prostate Cancer,Version 2.2023.
- Neal D. Shore,et al.Efficacy of olaparib (O) plus abiraterone (A) versus placebo (P) plus A in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with single homologous recombination repair gene mutations (HRRm) in the PROpel trial.ASCO-GU 2024,Abstract 165
- Maha H. A. Hussain, et al.BRCAAway: A randomized phase 2 trial of abiraterone, olaparib, or abiraterone + olaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) bearing homologous recombination-repair mutations (HRRm).ASCO-GU 2024.Abstract 19


