Editor's Note: Acute-on-Chronic Liver Failure (ACLF) is a complex syndrome characterized by acute deterioration of liver function on the background of chronic liver disease, accompanied by liver and/or extrahepatic organ failure. It poses a significant challenge in internal medicine due to its high short-term mortality rate of 50% to 90%. Early prediction of the risk of ACLF in patients and advancing the treatment window can effectively improve patient outcomes.Differences in etiology, underlying chronic liver diseases, and study populations across various countries and regions have led to varying definitions, diagnostic criteria, clinical classification, and prognostic assessments of ACLF. This creates difficulties for front-line clinicians in diagnosis and treatment. Recently, at the 32nd Annual Conference of the Asia-Pacific Association for the Study of the Liver (APASL), Professor Li Jun from the First Affiliated Hospital of Zhejiang University, China, was invited to present the latest progress of their ACLF early warning model based on a prospective, multicenter cohort study in China.
ACLF, or Acute-on-Chronic Liver Failure, is a complex condition with a high mortality rate under internal medicine treatment, due to its intricate causes and unclear pathogenesis. In Western populations, chronic liver disease is primarily caused by alcoholic cirrhosis (60.3%), with severe bacterial infections being the leading cause of ACLF [1]. In the Asia-Pacific region, however, hepatitis B virus (HBV) infection is the most common underlying disease. In those with HBV, factors like spontaneous activation of HBV, viral mutations, and drug resistance are major triggers for ACLF (59.1%) [2-3]. Therefore, conducting global multicenter, prospective studies and establishing unified ACLF diagnostic and prognostic scoring standards based on evidence-based medicine is an urgent need.
ACLF Diagnosis: Which of the Four Major Standards is Superior?
Currently, there is no universal definition or diagnostic criteria for ACLF internationally. Commonly used definitions include the European Association for the Study of the Liver ACLF criteria (EASL-ACLF), the Asia-Pacific Association for the Study of the Liver consensus (APASL-ACLF), the North American Consortium for the Study of End-Stage Liver Disease ACLF criteria (NACSELD-ACLF), and the Chinese Severe Hepatitis B Research Group ACLF criteria (COSSH-ACLF) (Figure 1).
1. EASL-ACLF defines it as a special syndrome in cirrhotic patients, emphasizing the importance of extrahepatic organ failure. It’s characterized by acute decompensation (AD) of liver functions, organ failure, and high short-term mortality. The occurrence of ascites, hepatic encephalopathy, gastrointestinal bleeding, and/or bacterial infections are defined as AD; some patients without a history of AD can also develop ACLF. Organ failure includes the liver, kidneys, brain, respiratory system, circulatory system, and coagulation functions.
2. NACSELD-ACLF defines ACLF as the presence of at least two severe extrahepatic organ failures, including shock, grade III-IV hepatic encephalopathy (HE), renal replacement therapy (RRT), or mechanical ventilation, on the basis of liver cirrhosis, with a higher short-term (30-day) mortality. This standard is only applicable to patients with ADC and infections and is less frequently used.
3. APASL-ACLF defines it as acute liver injury on the basis of chronic liver disease or cirrhosis, with a focus on liver failure. The main manifestations are jaundice [serum bilirubin ≥5 mg/dL (85 μmol/L)] and coagulation dysfunction [International Normalized Ratio (INR) ≥1.5 or prothrombin activity <40%], with complications like ascites and/or hepatic encephalopathy within 4 weeks, and high mortality within 28 days. The absence of extrahepatic organ failure does not affect the diagnosis.
4. COSSH-ACLF, based on a prospective multicenter cohort study in China, considers HBV-ACLF a clinical syndrome with high short-term mortality, characterized by acute deterioration of liver function and liver or extrahepatic organ failure, on the background of chronic liver disease (with or without cirrhosis) caused by HBV. This new standard increases the proportion of early-diagnosed ACLF patients by nearly 20%, addressing the inadequacy of the EASL-ACLF standard for the hepatitis B population.
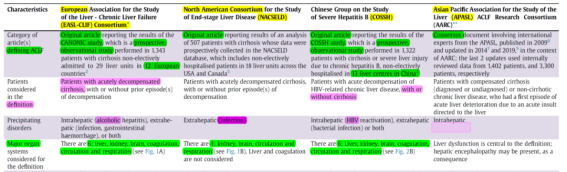
Figure 1. Comparison of Common ACLF Definitions and Diagnostic Criteria (Adapted from Presenter’s Slides)
Prognostic Assessment of ACLF: Which of the Five Scoring Systems is Stronger?
ACLF progresses rapidly, and early accurate assessment of its prognosis can provide clinicians with treatment plans to improve outcomes. Commonly used liver scores include the Child-Turcotte-Pugh score (CTP score), the Model for End-Stage Liver Disease (MELD) score, and the MELD-Na score. However, numerous studies have shown these scores to have poor sensitivity and specificity in predicting ACLF outcomes. In response, scholars from around the world, based on the aforementioned ACLF diagnostic criteria, have established and updated various prognostic scoring models for ACLF. These include the CLIF-C ACLF score, COSSH-ACLF score, COSSH-ACLF II score, APASL-AARC score, and NACSELD-ACLF score, among others (Figure 2).
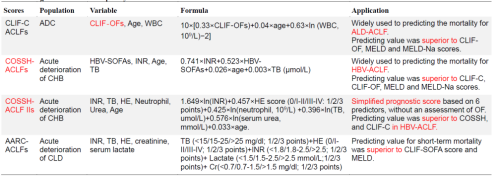
Figure 2. Comparison of Four Prognostic Scoring Systems for ACLF (Adapted from Presenter’s Slides)
ACLF Early Warning Assessment: Achieving Primary Prevention
Current research primarily focuses on the prognostic assessment of ACLF, with less emphasis on early warning assessment systems for its onset. The PREDICT study, a prospective, observational study from Europe [4], enrolled 1071 patients with acute decompensated cirrhosis (AD), divided into three groups: Pre-ACLF (n=218), UDC (n=233), and SDC (n=620). Over time, the weekly incidence of ACLF gradually increased in the Pre-ACLF subgroup. Events such as bacterial infections, severe alcoholic hepatitis, hemorrhagic shock, and toxemic encephalopathy were related to the progression of AD. Active prevention and treatment of these events could improve patient outcomes. However, the study did not successfully establish a model for accurately predicting the onset of ACLF.
Turning attention back to China, Professor Li Jun’s research group, leading 16 centers nationwide, conducted a prospective, multicenter, open large cohort study [5]. They established a China-specific standard (COSSH-ACLF) for diagnosing HBV-ACLF and a prognostic stratification scoring system suitable for the hepatitis B population. Based on the COSSH open research cohort, 1373 patients with hepatitis B cirrhosis experiencing acute decompensation or severe liver injury on the basis of chronic hepatitis B were prospectively enrolled. Results from LASSO-Cox regression and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) showed that total bilirubin (TB), INR, alanine aminotransferase (ALT), and ferritin were the best indicators for predicting ACLF within 7 days. Based on these indicators, the COSSH-onset-ACLF early diagnosis and warning model was developed [COSSH-onset-ACLFs = 0.101 × ln(ALT) + 0.819 × ln(TB) + 2.820 × ln(INR) + 0.016 × ln(ferritin)].
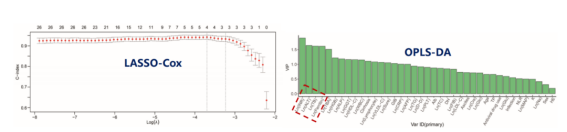
Figure 3. Results from LASSO-Cox Regression and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) (Adapted from Presenter’s Slides)
Analysis of the predictive efficiency of the scoring model revealed that the COSSH-onset-ACLFs model’s concordance index (C-index) for predicting ACLF occurrence at 7, 14, and 28 days (0.928, 0.925, 0.913) was significantly higher than that of the other five scores (CLIF-C ACLF-Ds, MELDs, MELD-Nas, COSSH-ACLFs, CLIF-C ACLFs, all with P values <0.001). Additionally, the rate of prediction error improvement was notably enhanced (Figure 4).
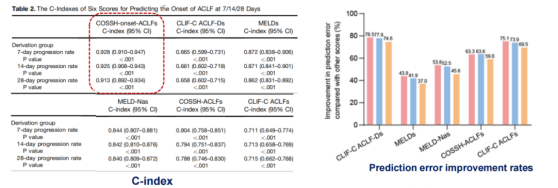
Figure 4. Analysis Results of the Concordance Index (C-index) (Adapted from Presenter’s Slides)
Time-dependent ROC analysis demonstrated that the COSSH-onset-ACLFs model had the highest area under the ROC curve (AUROC) at all time points (0.939, 0.939, 0.926), as shown in Figure 5.
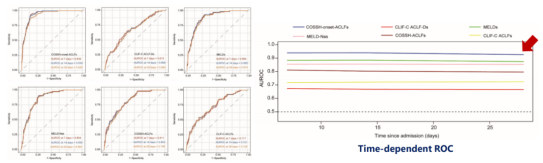
Figure 5. Time-Dependent ROC Analysis Results (Adapted from Presenter’s Slides)
The Probability Density Function (PDF) analysis assessed the scoring overlap between patients who developed ACLF and those who did not, across various scoring systems. The COSSH-onset-ACLFs model demonstrated the smallest overlap coefficient (30.2%, 30.3%, and 33.4% at 7, 14, and 28 days, respectively), as depicted in Figure 6.
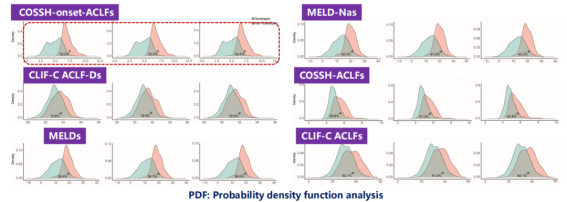
Figure 6. Results of the Probability Density Function (PDF) Analysis (Adapted from Presenter’s Slides)
The calibration analysis indicated excellent consistency between the probabilities of ACLF occurrence at 7, 14, and 28 days as predicted by COSSH-onset-ACLFs and the actual occurrence of ACLF. Risk stratification analysis revealed that the COSSH-onset-ACLFs model could classify all patients into high-risk (≥6.3) and low-risk (<6.3) groups for the development of ACLF at 7, 14, and 28 days; there was a significant difference in the risk of ACLF occurrence between the two groups at these time points (Figures 7 and 8). Notably, an external validation cohort of 391 non-ACLF cases further substantiated these findings.
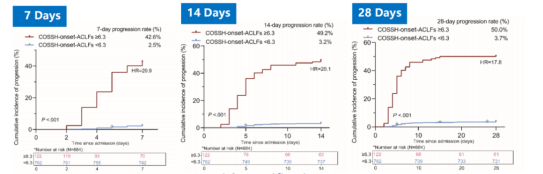
Figure 7. Results of Risk Stratification Analysis (Adapted from Presenter’s Slides)
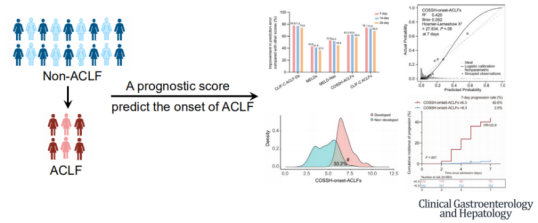
Figure 8. COSSH-onset-ACLF Early Diagnosis and Warning Model (Adapted from Presenter’s Slides)
Latest Developments in ACLF Treatment
Currently, there are no specific drugs or methods for the treatment of ACLF, and the main approach is comprehensive internal medicine treatment. Liver transplantation (LT) is the only effective treatment method. However, its implementation is limited due to factors such as the shortage of donor organs, varying quality of donated livers, patient immune rejection, high costs, and high mortality rate during the waiting period for transplantation [6-7]. Therefore, it is crucial to identify patients who still have a high risk of short-term mortality under standard treatment in the early stages of hospitalization and to determine the optimal timing for transplantation.
To address this, Professor Li Jun’s team utilized data from hospitalized patients with acute exacerbation of chronic hepatitis B in the COSSH open cohort (n=4577). They calculated the survival benefit rate to reflect the expected lifespan extension of patients undergoing LT compared to those not receiving LT, thereby identifying the high-benefit group for liver transplantation. The study results [8] showed that the 1-year survival rate of HBV-ACLF patients who underwent LT was significantly improved. Stratified analysis revealed that most ACLF patients (79.1%) had COSSH-ACLF IIs scores between 7 and 10, and these patients had a much higher net survival benefit from LT compared to patients with scores <7 or >10 (Figure 9).
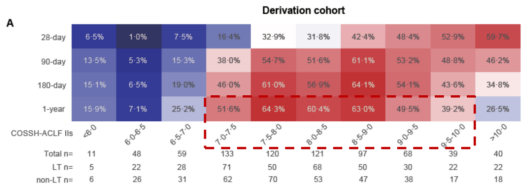
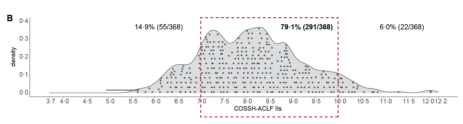
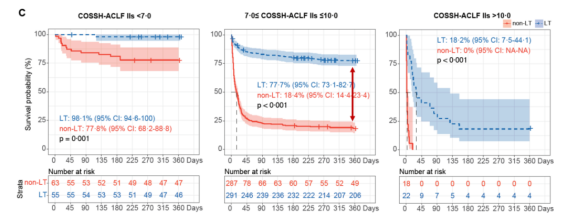
Figure 9. Survival Benefit Analysis for ACLF Patients (Adapted from Presenter’s Slides)
Conclusion
Due to the differences in etiology and triggers, there are numerous disparities and even controversies among Eastern and Western scholars regarding the definition, diagnostic criteria, and pathogenesis of ACLF. Clarifying the pathogenic process of ACLF and providing targeted treatment based on different mechanisms are crucial for reducing the mortality rate of ACLF. Current data indicate that the COSSH-onset-ACLFs early warning model, based on four simple clinical indicators, can accurately predict the occurrence of ACLF at 7, 14, and 28 days. This aids in the early and precise identification of high-risk ACLF patients, achieving primary disease prevention. For ACLF patients, those with a COSSH-ACLF II score of 7-10 points receive a higher net survival benefit from liver transplantation (LT).


