According to the reports from Phase III MARIPOSA and MARIPOSA-2 trials presented at the 2023 ESMO Presidential Symposium, Amivantamab combination therapy demonstrated clinically significant improvements in both first-line and second-line treatments compared to standard therapy (Abstract Numbers LBA14 and LBA15).

Byoung Chul Cho (South Korea), Zofia Piotrowska (Boston, USA), and Antonio Passaro (Milan, Italy) during the third Presidential Symposium at ESMO 2023.
A majority of EGFR-mutant (ex19del or L858R) advanced non-small cell lung cancer (NSCLC) patients who undergo third-generation EGFR-TKI, Osimertinib, inevitably develop resistance (Ther Adv Med Oncol. 2022;14:17588359221144099). The demand for new first-line and recurrent treatment options remains unmet. Results from two highly anticipated Phase III trials, MARIPOSA (for treatment-naive patients) and MARIPOSA-2 (for Osimertinib-treated recurrent patients), showed positive outcomes for EGFR-MET dual-specific antibody Amivantamab combination therapy, confirming previous data from the CHRYSALIS and CHRYSALIS-2 studies (J Clin Oncol. 2023;41:16_suppl:9134; J Clin Oncol. 2022;40:16_suppl:9006).
01 First-line Treatment in MARIPOSA Trial (Abstract Number LBA14)
In the MARIPOSA trial, compared to 429 patients receiving Osimertinib monotherapy, 429 patients receiving Amivantamab in combination with irreversible third-generation EGFR-TKI Lazertinib demonstrated a 30% reduction in the risk of disease progression or death (hazard ratio [HR] = 0.70; 95% confidence interval [CI]: 0.58–0.85; p < 0.001). The median progression-free survival (PFS) was 23.7 months (95% CI: 19.1–27.7) for the combination therapy and 16.6 months (95% CI: 14.8–18.5) for Osimertinib alone, with a median follow-up of 22.0 months.
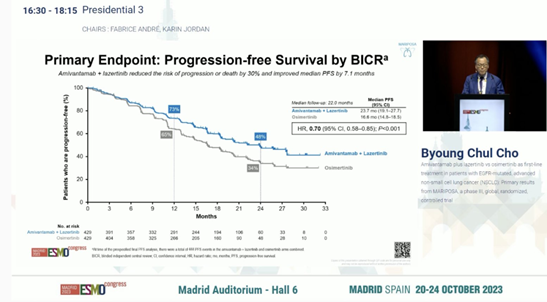
In the MARIPOSA trial, Amivantamab-Lazertinib showed improved PFS compared to Osimertinib.
The objective response rate (ORR) was similar between the two groups (86% for Amivantamab-Lazertinib vs. 85% for Osimertinib), but the median duration of response (DOR) was longer in the Amivantamab-Lazertinib group, at 25.8 months (95% CI: 20.1–not estimable) vs. 16.8 months (95% CI: 14.8–18.5) in the Osimertinib group.
In the mid-term overall survival (OS) analysis, the Amivantamab-Lazertinib group showed a trend of better outcomes than the Osimertinib group (HR = 0.80; 95% CI: 0.61–1.05; p = 0.11).
The Amivantamab-Lazertinib group experienced higher rates of EGFR and MET inhibition-related adverse events (AEs) compared to the Osimertinib group. Treatment-related adverse events leading to discontinuation occurred in 10% of patients in the Amivantamab-Lazertinib group and 3% in the Osimertinib group. Venous thromboembolism (VTE) occurred in 37% of patients in the Amivantamab-Lazertinib group and 9% in the Osimertinib group, leading the researchers to recommend prophylactic anticoagulation in the first 4 months of Amivantamab-Lazertinib treatment.
Zofia Piotrowska, Professor at Massachusetts General Hospital/Harvard Medical School, commented on the MARIPOSA study results, stating, “While we must weigh the benefits and increased toxicity of both drugs, and the practicality of adding intravenous Amivantamab (given every two weeks) to an oral treatment regimen, Amivantamab combination therapy shows clinically meaningful PFS improvement compared to current standard treatment. Skin toxicities, such as paronychia and rash, are more common with combination therapy. Although these side effects are mostly low-grade, they may impact patients’ quality of life, especially considering the long duration of treatment. Clinical practitioners need to balance these risks and the degree of clinical benefit when considering this regimen.”
02 Second-line Treatment in MARIPOSA-2 Trial (Abstract Number LBA15)
In the second-line treatment MARIPOSA-2 trial, patients receiving Amivantamab monotherapy with chemotherapy (n=131; HR=0.48; 95% CI: 0.36–0.64; p < 0.001) and Amivantamab-Lazertinib combination therapy with chemotherapy (n=263; HR=0.44; 95% CI: 0.35–0.56; p < 0.001) showed significantly improved PFS compared to those receiving chemotherapy alone (n=263). The median PFS for the Amivantamab-chemotherapy group, Amivantamab-Lazertinib-chemotherapy group, and chemotherapy-alone group were 6.3 months, 8.3 months, and 4.2 months, respectively.
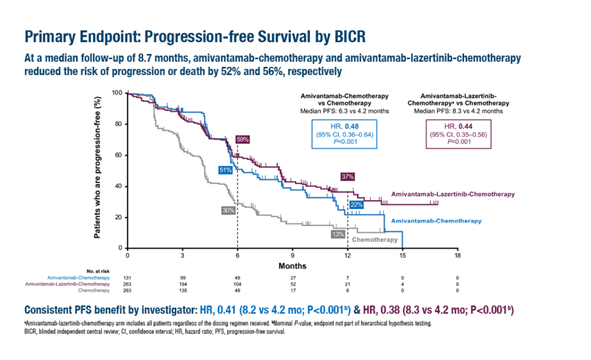
In the MARIPOSA-2 trial, both Amivantamab-chemotherapy and Amivantamab-Lazertinib-chemotherapy groups had significantly prolonged median PFS compared to the chemotherapy-alone group.
The ORR was 64% in the Amivantamab-chemotherapy group, 63% in the Amivantamab-Lazertinib-chemotherapy group, and 36% in the chemotherapy-alone group (p < 0.001).
Overall survival (OS) data are not mature yet, but interim analysis suggests that compared to chemotherapy, the OS hazard ratio was 0.77 (95% CI: 0.49–1.21) for Amivantamab-chemotherapy and 0.96 (95% CI: 0.67–1.35) for Amivantamab-Lazertinib-chemotherapy.
In the Amivantamab-chemotherapy and Amivantamab-Lazertinib-chemotherapy groups, the median intracranial PFS was 12.5 months and 12.8 months, respectively, compared to 8.3 months in the chemotherapy-alone group (HRs of 0.55 and 0.58; p = 0.001 and p < 0.001).
In the triplet regimen, ≥ grade 3 hematologic toxicity was common, including neutropenia (55%), thrombocytopenia (37%), and leukopenia (27%), requiring dosing adjustments. In the
Amivantamab-chemotherapy group, the most common ≥ grade 3 AEs were neutropenia (45%), leukopenia (20%), and thrombocytopenia (15%).
Based on these data, Piotrowska emphasized that the results of the MARIPOSA trial must be considered in conjunction with other new data, including the results of the FLAURA2 trial (NCT04035486) presented at the 2023 World Conference on Lung Cancer (WCLC). She noted that a comprehensive understanding of patient treatment is needed, taking into account various available treatment options and the best sequence, which may differ for individual patients. If Amivantamab-Lazertinib is used in the first line based on the MARIPOSA trial results, the Amivantamab combination regimens in MARIPOSA-2 may no longer be applicable for recurrence. If the first-line treatment is with the approved Osimertinib regimen, the consideration of Amivantamab combination therapy (with or without chemotherapy) as a second-line drug may be appropriate.
Reference :
1. Cho BC, et al. Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. ESMO Congress 2023, LBA14
2. Passaro A, et al. Amivantamab plus chemotherapy (with or without lazertinib) vs chemotherapy in EGFR-mutated advanced NSCLC after progression on osimertinib: MARIPOSA-2, a phase III, global, randomized, controlled trial. ESMO Congress 2023, LBA15

