At the 67th American Society of Hematology (ASH) Annual Meeting in 2025, a groundbreaking Late-Breaking Abstract (LBA) presented by Professor Phoebe Joy Ho (Royal Prince Alfred Hospital, Australia) signaled a potential paradigm shift in the treatment of multiple myeloma. The preliminary results from the Phase 1 inMMyCAR study of KLN-1010—the first in vivo anti-BCMA CAR-T therapy—demonstrated that deep molecular responses can be achieved without the complex logistics of ex vivo manufacturing or the toxicities associated with lymphodepleting chemotherapy.Mechanism Innovation: Direct In Vivo Generation
Traditional CAR-T therapies, while effective, are burdened by complex manufacturing processes involving T-cell collection, ex vivo engineering, and expansion. Furthermore, patients must undergo lymphodepleting chemotherapy to facilitate CAR-T expansion, which contributes to significant toxicity.
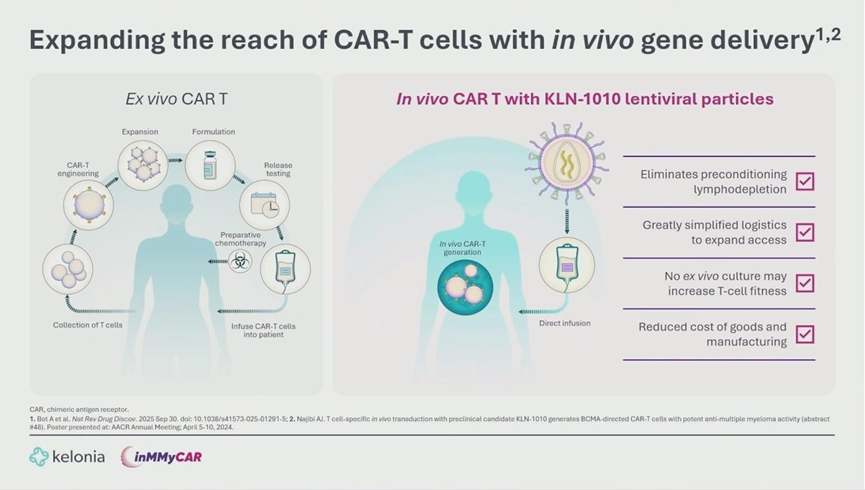
KLN-1010 circumvents these hurdles by delivering the CAR transgene directly into the patient’s body via an intravenous injection of a modified lentiviral vector (LVV).
- Precision Engineering: The vector utilizes a mutated VSV-G envelope to prevent non-specific binding (de-targeting) and incorporates an anti-CD3 scFv to specifically target T-cells (re-targeting).
- Key Advantages: This “off-the-shelf” approach eliminates the need for bridge therapy and preconditioning chemotherapy, preserves T-cell fitness by avoiding ex vivo culture exhaustion, and simplifies supply chain logistics.
Study Design and Baseline Characteristics
The inMMyCAR study (NCT07075185) is a multicenter, open-label, dose-escalation Phase 1 trial. The report covered the first four patients with Relapsed/Refractory Multiple Myeloma (RRMM).
- Patient Profile: Patients were aged 61-72 with high-risk cytogenetics. All were triple-class refractory (PI, IMiD, anti-CD38 mAb) and had received 3-5 prior lines of therapy. None had prior BCMA-targeted therapy.
- Dosing: Three patients received Dose Level 1 (2×10⁷ IU/kg), and due to high potency, the fourth patient received a lower dose (DL-1).
Efficacy: 100% MRD Negativity
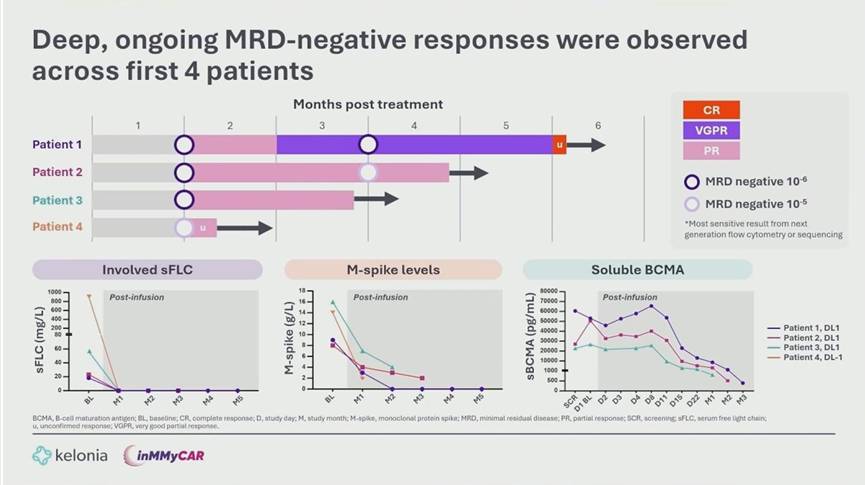
Despite being an early-phase safety study, KLN-1010 demonstrated profound efficacy:
- MRD Status: All 4 patients achieved Minimal Residual Disease (MRD) negativity (10⁻⁶ for 3 patients, 10⁻⁵ for patient 4 awaiting sequencing results) by Month 1 post-treatment. This status was sustained at the Month 3 assessment for the first two patients.
- Clinical Response: As of the data cutoff, one patient achieved a Complete Response (CR) by Month 5, while others remain in Partial Response (PR) with rapid normalization of serum free light chains, suggesting responses will deepen over time.
Pharmacokinetics: Robust Expansion Without Chemotherapy
A critical finding was the robust expansion of CAR-T cells in the complete absence of lymphodepletion.
- Cellular Kinetics: Digital PCR analysis showed peak vector copy numbers comparable to approved ex vivo therapies (e.g., cilta-cel).
- Phenotype: The in vivo generated CAR-T cells were enriched for central memory (Tcm) and stem-like (Tscm) phenotypes. This profile is associated with superior persistence and durability compared to the exhausted phenotypes often seen in ex vivo manufacturing.
Safety Profile: Superior Tolerability
The safety data highlighted a distinct advantage over traditional CAR-T therapies:
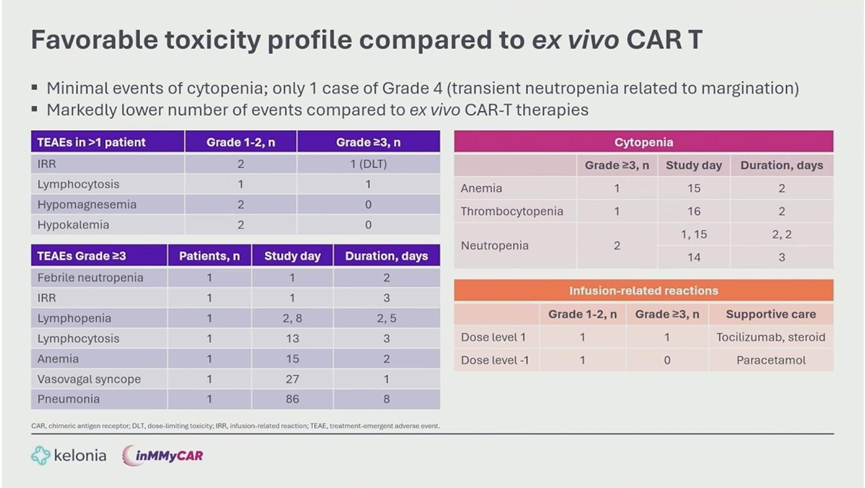
- CRS: Cytokine Release Syndrome occurred in 3 of 4 patients but was limited to Grade 1-2. Onset was late (median Day 10), and all cases resolved quickly with standard management.
- Neurotoxicity: No cases of ICANS or delayed neurotoxicity were observed.
- Hematology: Unlike ex vivo CAR-T, there were no prolonged high-grade cytopenias. One transient Grade 4 neutropenia resolved within 16 hours.
Conclusion
Professor Phoebe Joy Ho concluded that the inMMyCAR study provides successful Proof-of-Concept for in vivo CAR-T therapy. KLN-1010 successfully generated functional, persistent CAR-T cells within the patient’s body, achieving deep efficacy (100% early MRD negativity) with a favorable safety profile that supports outpatient administration. As dose expansion continues, this therapy holds the promise of democratizing access to CAR-T treatment by removing manufacturing bottlenecks and reducing systemic toxicity.