
The CheckMate 8HW trial, presented at the ASCO Gastrointestinal Cancers Symposium by Thierry Andre, is a randomized, multicenter, open-label phase 3 study evaluating the efficacy and safety of nivolumab (NIVO) in combination with ipilimumab (IPI) compared to nivolumab alone in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC). The study aimed to establish whether the combination therapy provides superior outcomes in terms of progression-free survival (PFS), overall response rate (ORR), and health-related quality of life (HRQoL).Patient Characteristics
The trial included 1,059 patients randomized into three treatment arms: nivolumab monotherapy, nivolumab plus ipilimumab, and investigator’s choice chemotherapy (mFOLFOX6 or FOLFIRI with or without bevacizumab or cetuximab). Eligibility criteria required histologically confirmed, unresectable or metastatic CRC with centrally confirmed MSI-H/dMMR status and an ECOG performance status of 0 or 1. The median follow-up duration at the data cutoff was 47.0 months (range, 16.7-60.5 months).
Baseline characteristics showed a median patient age of 62 years in the combination therapy group and 63 years in the nivolumab monotherapy group. Sex distribution was balanced, with 46% of patients being male and 54% female. Liver and peritoneal metastases were present in 40% of the nivolumab plus ipilimumab group, while 42% had liver metastases in the monotherapy group. BRAF and KRAS/NRAS mutations were assessed, with approximately 30% of patients harboring BRAF mutations.
Efficacy Outcomes
Nivolumab plus ipilimumab demonstrated a statistically significant improvement in PFS compared to nivolumab monotherapy. The median PFS was not reached in the combination therapy group versus 39.3 months in the monotherapy group, with a hazard ratio (HR) of 0.63. At 12 months, PFS rates were 76% for nivolumab plus ipilimumab versus 63% for nivolumab alone. At 24 months, PFS rates were 71% and 56%, respectively, and by 36 months, the rates were 68% and 51%, respectively (HR, 0.62; 95% CI, 0.48-0.81; p = 0.0003). These findings highlight the sustained benefit of combination therapy across all time points.
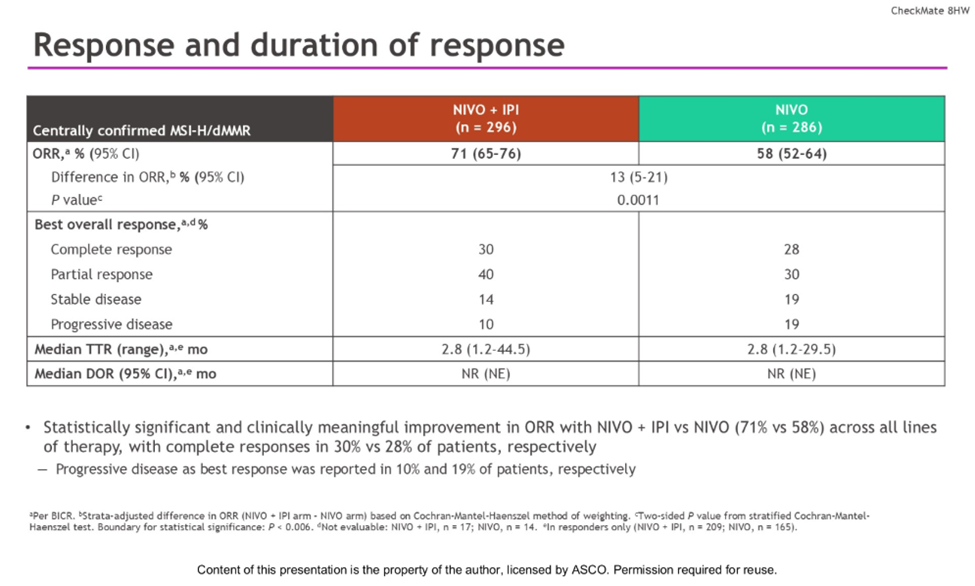
The overall response rate (ORR) was also superior in the combination arm at 71% (95% CI, 65-76%) compared to 58% (95% CI, 52-64%) in the nivolumab monotherapy arm. The difference in ORR was 13% (95% CI, 5-21%), with a statistically significant p-value of 0.0011. Complete responses were observed in 30% of patients in the combination group versus 28% in the monotherapy group, while partial responses were 40% and 30%, respectively. The median time to response was 2.8 months in both treatment arms.
Health-Related Quality of Life
Health-related quality of life (HRQoL) improvements were assessed using the EORTC QLQ-C30 Global Health Status subscale. Mean change from baseline scores was consistently positive in both arms, with the nivolumab plus ipilimumab group reaching the prespecified threshold for meaningful change by week 21. These findings suggest that combination therapy maintains or enhances patient well-being during treatment.
Safety and Adverse Events
The safety profile of nivolumab plus ipilimumab was consistent with known immune-related adverse events (AEs). Treatment-related adverse events (TRAEs) of any grade were reported in 81% of patients in the combination group and 71% in the monotherapy group. Grade 3/4 TRAEs occurred in 22% of combination therapy patients versus 14% in the monotherapy group. The most common TRAEs included pruritus (26%), diarrhea (20%), hypothyroidism (17%), asthenia (16%), and fatigue (12%). Serious TRAEs were reported in 18% of patients receiving combination therapy compared to 8% in the monotherapy group, with 6% of combination therapy patients discontinuing treatment due to adverse effects.
Immune-mediated adverse events (IMAEs) included hypothyroidism (18% vs. 9%), hyperthyroidism (12% vs. 5%), and adrenal insufficiency (10% vs. 3%) in the combination versus monotherapy groups, respectively. Pneumonitis was reported in 2% of patients receiving combination therapy and 1% in those receiving monotherapy.
Treatment Disposition and Exposure
A total of 45% of patients in the combination therapy arm completed treatment, while 57% discontinued due to disease progression, adverse events, or other reasons. The median duration of treatment was 20.5 months in the nivolumab plus ipilimumab arm compared to 16.4 months in the nivolumab monotherapy arm. Median dose exposure was 23 doses of nivolumab and four doses of ipilimumab in the combination group, while nivolumab monotherapy patients received a median of 21 doses.
Conclusion
The CheckMate 8HW trial demonstrated that nivolumab plus ipilimumab provides a clinically meaningful improvement in progression-free survival and overall response rate compared to nivolumab alone in patients with MSI-H/dMMR mCRC. These benefits were consistent across subgroups and accompanied by improvements in health-related quality of life. While the combination therapy was associated with higher rates of immune-related adverse events, the overall safety profile remained manageable. Given these findings, nivolumab plus ipilimumab represents a promising standard-of-care option for MSI-H/dMMR metastatic colorectal cancer.


