In May 2023, a study led by Professor Rong Fu from Tianjin Medical University General Hospital was published in the prestigious international academic journal —— British Journal of Haematology(IF=8.615). The title of the study is "Gene therapy with B-cell maturation antigen/CD3 bispecific antibody encoding plasmid DNA for treating multiple myeloma". The study introduce the potential of gene therapy as a viable alternative to conventional BsAb therapies.
Multiple myeloma (MM) remains a significant challenge in the field of hematology, characterized by its incurability and frequent relapses despite advancements in treatment. In the quest for more effective therapies, bispecific antibodies (BsAbs) targeting B-cell maturation antigen (BCMA) have emerged as a promising strategy. However, the complexity and cost of BsAb production pose significant barriers. Addressing this gap, the study by Fengping Peng and colleagues explores an innovative gene therapy approach, utilizing plasmid DNA encoding BCMA/CD3 BsAbs to offer a potential breakthrough in MM treatment.
This study aimed to evaluate the efficacy of a novel gene therapy using a plasmid DNA encoding BCMA/CD3 BsAb in treating MM. By harnessing gene therapy, the research sought to surmount the challenges of conventional BsAb therapies, such as high production costs and complex manufacturing processes.
The study utilized a xenograft mouse model, implanting NOD-SCID mice with RPMI-8226 MM cells. The mice were divided into three groups, each receiving distinct treatments: an electroporation-enhanced intramuscular injection of pCAG-BCMA/CD3 plasmid, BCMA/CD3 BsAb, and a control BsAb (hen egg lysozyme/CD3). The effectiveness of these treatments was measured through tumor size, blood analyses for liver and kidney toxicity, interferon γ levels, and detailed tissue examinations.
Key data from the study included:
– Enhanced T-cell activation and effective lysis of MM cells by BCMA/CD3 BsAbs.
– Higher serum levels of BsAbs following gene therapy compared to BiTE-Fc fusion.
– Significant inhibition of tumor growth in both gene therapy and BCMA/CD3 BsAb treatment groups.
– Minimal toxicity observed in the treatment groups.
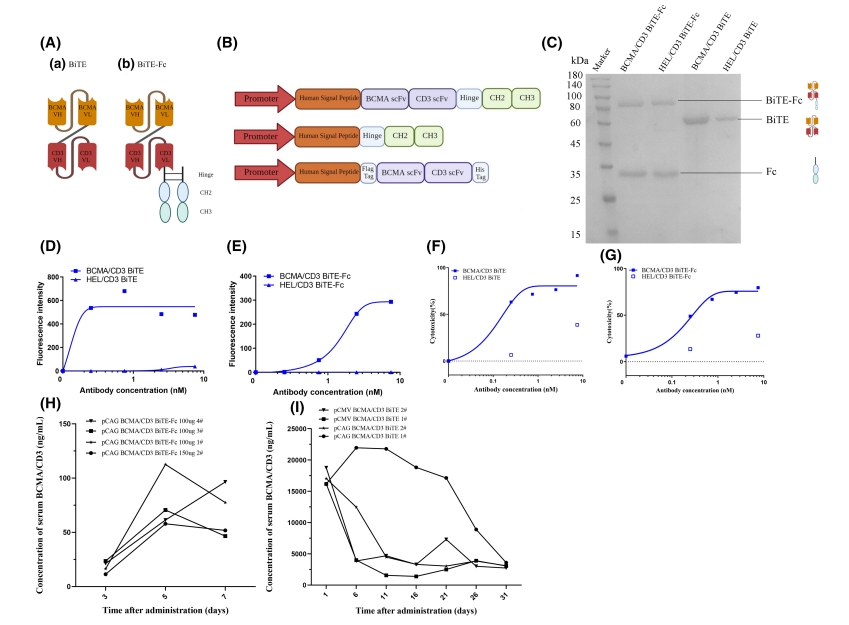
Figure 1: Characterization of BCMA/CD3 bispecific antibodies (BsAbs).
(Br J Haematol . 2023 May;201(3):417-421. )
The study’s findings demonstrated that:
– Gene therapy effectively delivered BCMA/CD3 BsAbs, significantly inhibiting tumor growth in the MM model.
– The safety profile of the gene therapy was favorable, with limited toxicity.
– Enhanced immune response was evident through increased T-cell infiltration and sustained interferon γ levels.
The study marks a pivotal step in MM treatment, showcasing the potential of gene therapy as a viable alternative to conventional BsAb therapies. By successfully demonstrating the efficacy and safety of plasmid-based BCMA/CD3 BsAb delivery, this research opens new horizons in MM therapy. It offers hope for more accessible and cost-effective treatment options, potentially transforming the management of MM and improving patient outcomes. This innovative approach warrants further investigation and paves the way for clinical trials, bringing us closer to a new era in the battle against MM.


