Editor's Note: Primary central nervous system lymphoma (PCNSL) is a relatively rare malignant tumor of the central nervous system, belonging to extranodal non-Hodgkin lymphoma. Due to factors such as the blood-brain barrier, achieving satisfactory treatment outcomes with traditional methods is challenging. The prognosis for PCNSL is poorer compared to systemic non-Hodgkin lymphoma. With advancements in PCNSL genetics and molecular research, there is optimism about the potential of small molecule and targeted therapies to improve outcomes with fewer adverse effects. At the recently concluded 28th European Hematology Association (EHA) Congress, lymphoma research continued to captivate attention. Dr. Zhou Daobin's team from Peking Union Medical College Hospital, China, presented a study (abstract number: P1165) that evaluated the efficacy and safety of the pomalidomide-obinutuzumab-lenalidomide (POR) regimen followed by high-dose methotrexate in newly diagnosed PCNSL patients. To gain in-depth insights into this study, Oncology Frontier invited Dr. Zhou to provide a detailed interpretation and analysis, summarized below.
Background
High-dose methotrexate (MTX) is widely used in frontline treatment for primary central nervous system lymphoma (PCNSL). Clinical data have shown that the new generation immunomodulator pomalidomide and Bruton kinase inhibitor obinutuzumab can be used to treat relapsed/refractory PCNSL, both having good blood-brain barrier permeability and potential synergistic effects.
Objectives
To evaluate the efficacy and safety of the sequential high-dose methotrexate regimen with pomalidomide, obinutuzumab, and lenalidomide (POR) in newly diagnosed PCNSL patients, we conducted a prospective, single-arm, open-label phase II clinical study.
Methods
Inclusion criteria were untreated PCNSL patients aged 18-70 years, HIV-Ab negative, with assessable lesions and sufficient organ and bone marrow function. All patients received four cycles of the PRO regimen: pomalidomide 4 mg/day orally on days 1-14, obinutuzumab 150 mg/day orally on days 1-21, and lenalidomide 375 mg/m2 intravenously on day 1, with a 21-day cycle. Patients who responded to the 4-cycle POR regimen (achieving complete or partial response) received two cycles of the ROM regimen: lenalidomide 375 mg/m2 intravenously on day 1, obinutuzumab 150 mg/day orally on days 1-21, and methotrexate 3.5 g/m2 intravenously over 4 hours on day 1, with a 21-day cycle. The primary endpoint was the overall response rate (ORR) after the four-cycle PRO regimen. The study is registered under NCT 05390749.
Results
As of February 15, 2023, 13 patients were enrolled. The median age was 58 years, with 5 patients having ocular involvement, 1 patient with cerebrospinal fluid (CSF) involvement, 5 patients having an IELSG score of 0-1, and 8 patients showing MYD88 L265P mutation in ctDNA analysis.
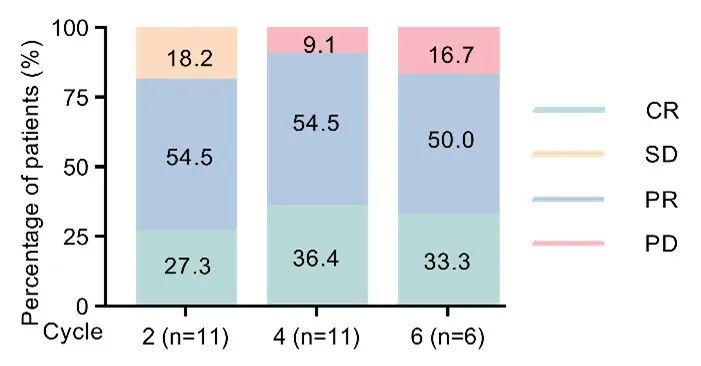
Efficacy Data:Among the 11 patients evaluated for efficacy (see Figure 1), the results showed an ORR/CRR of 81.8%/27.3% after the completion of two cycles of POR and 90.9%/36.4% after the completion of four cycles of POR. At a median follow-up of 4 months, only 1 patient experienced relapse, and median progression-free survival (PFS) and overall survival (OS) were not reached.
Stay tuned for further updates and insights from “Oncology Insight” on groundbreaking studies in the field of hematology.
Safety Data:Safety assessment was conducted for 12 patients who completed at least one cycle of treatment. Overall safety was manageable, with no unexpected adverse reactions. The main hematologic toxicities included neutropenia (n=8), leukopenia (n=8), anemia (n=5), and thrombocytopenia (n=4). Non-hematologic toxicities primarily included rash, infections, and elevated ALT, with infections mainly attributed to COVID-19. Until the data collection time, there were no off-target BTKi-related atrial fibrillation or severe bleeding events.
Researcher’s Comments:
This study attempted a chemotherapy-free induction strategy to reduce the chemotherapy cycle, exploring the efficacy and safety of the pomalidomide, obinutuzumab, lenalidomide (POR) regimen followed by RO-MTX in the treatment of newly diagnosed PCNSL. This is the first study investigating a chemo-free induction regimen for newly diagnosed PCNSL patients. In this study, the POR regimen demonstrated a high response rate and overall good safety, providing preliminary evidence of the treatment potential of chemotherapy-free induction for newly diagnosed PCNSL.
Original Article Link:
Yan Zhang, Chao Chen, Danqing Zhao, Zhe Zhuang, Chong Wei, Wei Wang, Wei Zhang, Dao-bin Zhou. Preliminary findings of a phase Ⅱ study of a chemo-free combination of pomalidomide, orelabrutinib, rituximab with sequential high-dose methotrexate in newly diagnosed primary CNS lymphoma. EHA 2023 Abstract P1165
TAG: EHA 2023, Voice of China,Hematological Malignancy, PCNSL


