
Editor’s Note: Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare and life-threatening blood disorder. Due to the complexity of complement biology and various pathogenic pathways, there remains a significant unmet clinical need, urgently requiring next-generation drugs with superior efficacy and dosing convenience compared to current therapies. At the recently held 65th American Society of Hematology (ASH) Annual Meeting, Prof. Fengkui Zhang from the Hematology Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology) and Prof. Bing Han’s team from Peking Union Medical College Hospital presented an oral report (Abstract: 572). The study explored the safety and effectiveness of the dual-function C5 antibody/H factor fusion protein KP104 in PNH patients who had not received complement inhibitor treatment. This research brings hope to address this challenging issue and has been selected as a highlight of “ASH 2024.” The summarized details are presented below in this issue of “Hematology Insight.”

Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and life-threatening hematologic disease characterized by intravascular and extravascular hemolysis (IVH and EVH) mediated by complement activation through both the terminal and alternative pathways (TP and AP). The current treatment options for PNH are limited to addressing only a single complement pathway, resulting in either unresolved EVH with TP inhibitors or potentially exposing patients to the risk of life-threatening breakthrough hemolysis with AP inhibitors (Notaro et al. N Engl J Med 2022;387:160-6). KP104 is an innovative fusion protein comprised of a humanized anti-C5 mAb and the functional domain of complement regulator factor H to inhibit both TP and AP activation. We report here the interim results from a phase 2 study of KP104 in complement inhibitor-naïve PNH patients.
Methods
This open-label Phase 2 clinical trial (NCT05476887) aims to assess the efficacy, safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of KP104 in patients diagnosed with PNH who have not received prior complement inhibitors. The primary objectives of the study are to evaluate key clinical markers including lactate dehydrogenase (LDH) and hemoglobin (Hgb) levels, transfusion requirements, and FACIT-fatigue scores. The first part of the study consists of three cohorts, with an enrollment of 6 patients per cohort. The study includes an initial treatment period of 12 or 13 weeks of KP104 dosing regimen, followed by a 9-month extension treatment period. The specific dosing regimens for each cohort are as follows:
Cohort 1: 1,200 mg IV (Day 1) + 720 mg SC QW (starting Day 8)
Cohort 2: 2,400 mg IV (Day 1) + 1,920 mg SC Q2W (starting Day 8)
Cohort 3: 3,600 mg IV (Day 1) + 2,880 mg SC Q2W (starting Day 8)
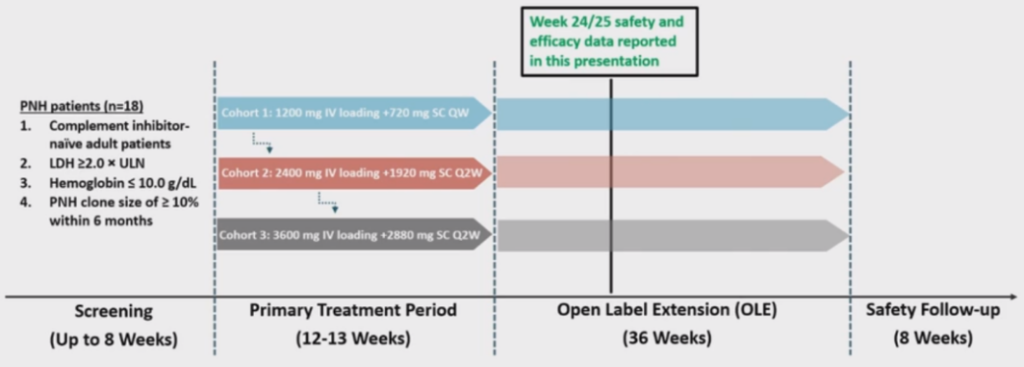
Figure 1. Study Design
Results
Interim results include data from 15 patients who completed 16/17 weeks of treatment (6 patients each from Cohort 1 and 2, and 3 from Cohort 3), as of the data cutoff on July 12th, 2023. Among these patients (4 males and 11 females, mean (±SD) age 33(±12) years, a median of 3 transfusions were required 12 months prior to the study and 9 patients were previously diagnosed with aplastic anemia. Baseline mean (SD) Hgb and LDH levels were 7.0 (±1.5) g/dL and 1,824 (±512) U/L, respectively.
By week 16/17, all 15 patients demonstrated positive clinical improvements. Mean (SD) Hgb levels increased by 4.9 (±1.7) g/dL, 5.8 (±1.6) g/dL, and 5.8 (±2.8) g/dL over baseline, and mean (SD) LDH levels reduced by 88.0 (±3.67) %, 83.5 (±7.45) %, and 89.5 (±4.05) % over baseline for Cohort 1, 2 and 3, respectively. Additionally, 9/15 (60%) patients achieved hemoglobin levels ≥12 g/dL (3/6, 5/6 and 1/3 for Cohort 1, 2 and 3, respectively), and all 15 patients had LDH levels below 1.5xULN. None of the 15 patients required transfusions or encountered any thromboembolic events after receiving KP104 treatment. Positive changes were observed in absolute reticulocyte count and bilirubin levels, along with significant enhancements in FACIT-fatigue scores seen in all 3 cohorts, with mean (SD) increases of 13.8 (±7.91), 12.8 (±9.15), and 9.3 (±10.69) for Cohorts 1, 2, and 3, respectively. Dose-dependent reductions in two PD biomarkers, C3b deposition and free C5 levels, further confirmed KP104’s dual mechanism of AP and TP inhibition.
KP104 was well tolerated, with no serious adverse events or treatment-emergent adverse events (TEAEs) that led to drug discontinuation or study withdrawal. 10/15 (67%) patients reported at least one mild or moderate TEAE, with no observed dose dependency. The most frequently reported TEAEs included transient injection site induration, headache, and COVID-19 infection, all of which were promptly or duly resolved. A single occurrence of clinical breakthrough hemolysis, observed in the lowest dose cohort and concurrent with an episode of gastroenteritis, was quickly resolved after an extra dose.
Table 1. Patient Baseline Characteristics

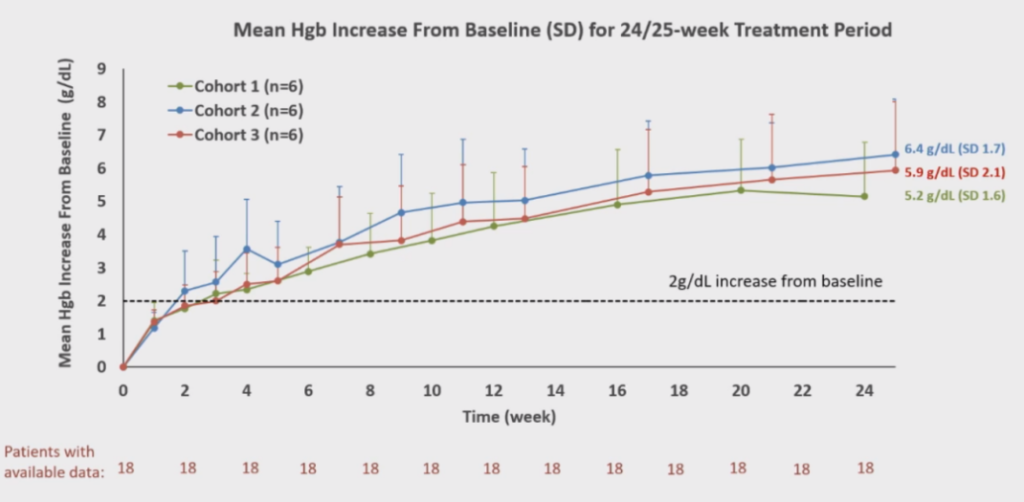
Table 2. Changes in Average Hgb Levels
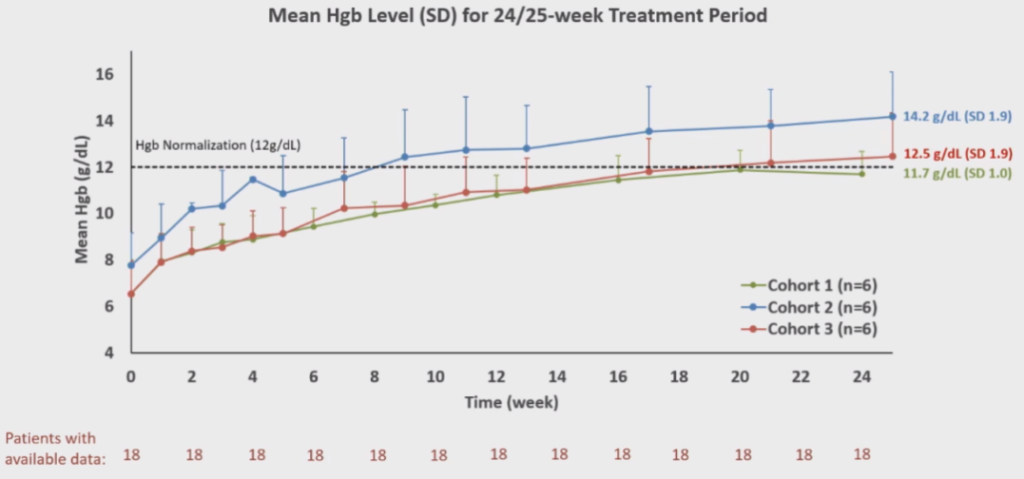
Table 3. Average Hgb Levels

Table 4. Average LDH Levels
Conclusion
The interim results of this phase 2 study demonstrate the safety and efficacy of KP104 in effectively controlling IVH and EVH in complement inhibitor-naïve PNH patients. The findings provide compelling clinical evidence that a bifunctional inhibitor targeting the AP and TP can properly address the current treatment gaps associated with single complement pathway inhibitors, potentially eliminating the need for cumbersome and costly combination therapies (Notaro et al. N Engl J Med 2022;387:160-6).
The researchers stated:
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare and life-threatening blood disorder caused by the overactivity of the complement system, which belongs to the innate immune system. PNH is characterized by red blood cell destruction, thrombosis, and impaired bone marrow function, affecting 1 to 5 individuals per million. Due to the complexity of complement biology and various pathogenic pathways in PNH, there remains a significant unmet clinical need for next-generation drugs with better efficacy and dosing convenience compared to current therapies. Current treatments include C5 inhibitors, which fail to address extravascular hemolysis (EVH) associated with alternative pathways, or C3 inhibitors, which may resolve EVH but face challenges in fully blocking downstream C5, leading to life-threatening breakthrough hemolysis.
KP104, developed by the SciClone Pharmaceuticals team led by Professor Wenru Song, is a globally pioneering dual-function complement biologic with a unique mechanism of action. It can selectively inhibit both the complement alternative pathway and terminal pathway, offering a powerful synergistic mechanism and potentially more selective precision in treating complement-mediated diseases. In our Phase II study presented at the ASH conference for PNH patients who had not received complement inhibitor treatment, positive results were observed. Eighteen patients completed 24/25 weeks of treatment, and mid-term analysis indicated that KP104 is safe, well-tolerated, and successfully met all major and secondary endpoints. KP104 effectively controlled intravascular and extravascular hemolysis, with all 18 patients experiencing an increase in hemoglobin by ≥2 g/dL compared to baseline without requiring transfusions. Moreover, most patients achieved normal ranges of hemoglobin and LDH levels. The mid-term results support advancing KP104 to Phase III clinical trials as a potentially optimal and safe treatment for PNH patients, addressing the current unmet clinical needs. Notably, this oral presentation on KP104 not only made it to the “ASH Highlights 2024” but also received recognition at the ASH President’s Symposium, securing the top position on the “2023 Novel PNH Inhibitors List.”

Professor Fengkui Zhang:
– **Degrees:** Ph.D.
– **Professional Titles:** Chief Physician, Professor, Doctoral Supervisor
– **Affiliation:** Hematology Hospital, Chinese Academy of Medical Sciences (Institute of Hematology)
– **Leadership Roles:**
– Director of the Anemia Diagnosis and Treatment Center, Hematology Hospital, Chinese Academy of Medical Sciences (Institute of Hematology)
– Director of the National New Drug Clinical Trial Institution, Hematology Hospital, Chinese Academy of Medical Sciences (Institute of Hematology)
– **Research Focus:** Clinical and experimental research on hematopoietic system red blood cell diseases
– **Pioneering Contributions:**
– Pioneered systematic reports on Large Granular Lymphocyte Leukemia in China
– Investigated and reported on congenital red blood cell disorders causing abnormal erythropoiesis
– Explored genetic instability in hematopoietic cells in patients with bone marrow failure
– Led the use of ATG combined with CsA and high-dose cyclophosphamide as a first-line treatment for bone marrow failure
– **Editorial Roles:**
– Deputy Editor-in-Chief of the “Chinese Journal of Hematology”
– Editorial board member of various hematology specialized journals
– **Research Contributions:**
– Principal investigator and participant in numerous research projects
– Published over ninety research papers
– **Awards and Recognitions:**
– Recipient of several awards, including recognition as an outstanding worker, excellent Communist Party member, outstanding teacher at the Medical Academy, and the “May 1st Labor Medal” in Tianjin
Professor Zhang’s extensive expertise, leadership, and contributions to the field of hematology underscore his commitment to advancing knowledge and improving the diagnosis and treatment of hematologic disorders.

Professor Bing Han:
– **Degrees:** Ph.D.
– **Professional Titles:** Chief Physician, Professor, Doctoral Supervisor
– **Affiliation:** Chief of the Hematology Department, Peking Union Medical College Hospital
– **Leadership Roles:**
– Leader of the Red Blood Cell Disease Group, Hematology Department, Peking Union Medical College Hospital
– Core Member of the International PNH Interest Group (IPIG)
– Deputy Director of the Red Blood Cell Disease Group, Chinese Medical Association Hematology Branch
– Head of the PNH Group, Chinese Medical Association Hematology Branch Rare Disease Group
– Deputy Director of the Academic Working Committee on Red Blood Cell Diseases, Second Committee of the Hematology Branch of the Chinese Medical Association Geriatrics Society
– Deputy Group Leader of the Second Committee of the MDS and MPN Working Group, Chinese Anti-Cancer Association Hematology Oncology Professional Committee
– Deputy Group Leader of the MDS Disease Group, Chinese Women Doctors Association
– Deputy Director of the Red Blood Cell Committee, Beijing Cancer Prevention and Treatment Society
– Deputy Director of the Cytology Professional Committee, Bai Qiu’en Spirit Research Association Laboratory Medicine Branch
– Deputy Director of the Red Blood Cell Disease Diagnosis Committee, Chinese Medical Doctor Association Laboratory Medicine Branch
– Standing Committee Member, Chinese Medical Association Geriatrics Hematology Branch
– Executive Director, World Federation of Chinese Medicine Societies
– Committee Member, Beijing Rare Disease Society
Professor Han’s diverse leadership roles across various medical associations and committees highlight her significant contributions to hematology research, rare disease advocacy, and medical professional organizations.


