【Editor's Note】 At the 2025 American Society of Hematology (ASH) Annual Meeting, Professor Wojciech Jurczak from the Maria Sklodowska-Curie National Research Institute of Oncology (Poland) presented the highly anticipated results of a global Phase III clinical trial. This study is the first head-to-head comparison evaluating the efficacy and safety of the non-covalent BTK inhibitor, pirtobrutinib, against the standard immunochemotherapy regimen of bendamustine plus rituximab (BR) in patients with treatment-naive chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).01. Background: Filling the Gap in First-Line Treatment
While covalent BTK inhibitors have transformed CLL management, Phase III data for non-covalent inhibitors in treatment-naive patients has been absent. Pirtobrutinib, a highly selective, reversible (non-covalent) BTK inhibitor, previously showed efficacy in relapsed/refractory settings, leading to this pivotal study.
The trial enrolled 282 treatment-naive CLL/SLL patients (median age 65-66 years), randomized 1:1 to receive either pirtobrutinib or the BR regimen. Notably, the study design allowed for crossover: patients in the BR arm could switch to pirtobrutinib upon confirmed disease progression.
02. Primary Endpoint: A Historic Hazard Ratio of 0.20
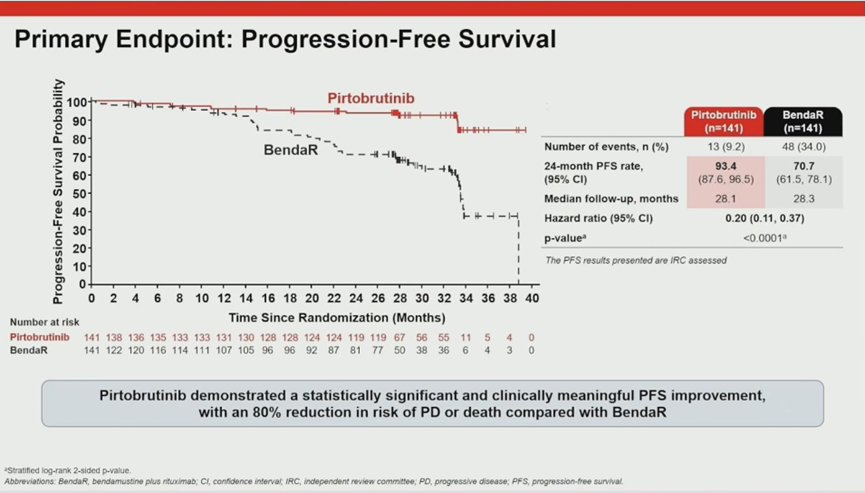
The study met its primary endpoint with overwhelming statistical significance, establishing a new benchmark for efficacy.
- PFS Results: The 24-month Progression-Free Survival (PFS) rate was 93% for the pirtobrutinib arm compared to 71% for the BR arm.
- Risk Reduction: The Hazard Ratio (HR) was 0.20 (P<0.0001). This translates to an 80% reduction in the risk of disease progression or death for patients treated with pirtobrutinib compared to immunochemotherapy.
- Consistency: Subgroup analyses confirmed that these benefits were consistent across all stratifications, including age and IGHV mutation status.
Prof. Jurczak highlighted that an HR of 0.20 represents one of the most significant treatment effects ever observed in comparisons between BTK inhibitors and BR regimens.
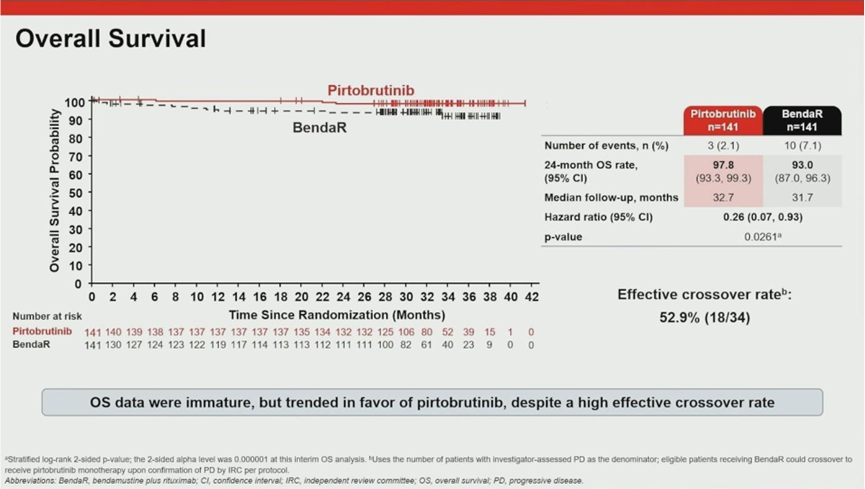
03. Overall Survival: Benefit Despite Crossover
A strong trend toward improved overall survival (OS) was observed, despite the fact that 53% of patients in the BR arm eventually crossed over to receive pirtobrutinib.
- Survival Rates: The 24-month OS rate was 98% in the pirtobrutinib arm versus 93% in the BR arm.
- Interim HR: The interim analysis showed an OS Hazard Ratio of 0.26.
These data suggest that early initiation of pirtobrutinib offers long-term survival advantages that rescue therapy at the time of progression cannot fully replicate.
04. Safety Profile: Superior Tolerability
The safety analysis underscored pirtobrutinib’s suitability for long-term use. Despite a much longer median treatment duration (32.3 months) compared to the fixed-duration BR arm (6 cycles), pirtobrutinib showed excellent tolerability.
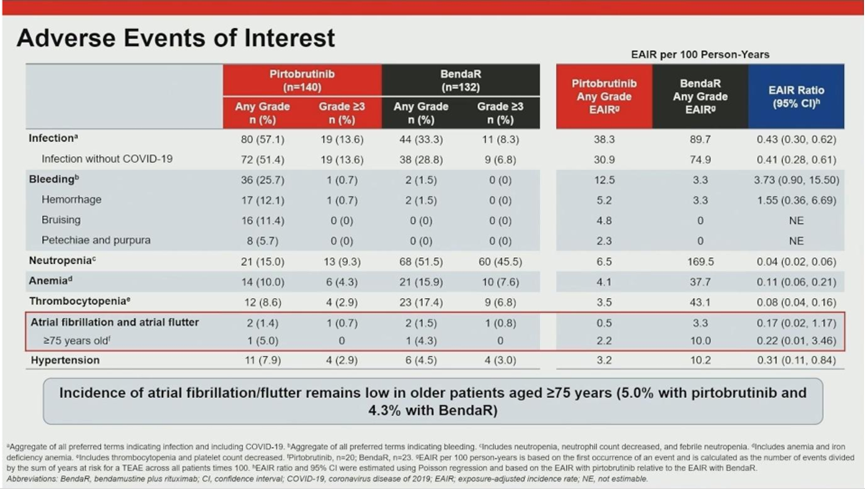
05. Conclusion and Clinical Implications
Prof. Jurczak concluded that pirtobrutinib has demonstrated superior PFS, a profound treatment effect size, and excellent tolerability compared to standard immunochemotherapy. It is poised to become a new standard of care for treatment-naive CLL, particularly for elderly or frail patients.
Expert Insight on Treatment Selection: During the discussion, Prof. Jurczak offered a nuanced perspective on choosing between “Fixed Duration” and “Continuous Therapy”:
- For Young/Fit Patients: Fixed-duration regimens may be preferred to allow a “return to normal life” after treatment.
- For Elderly/Frail Patients: Continuous therapy with pirtobrutinib is highly valuable. These patients often tolerate the intense monitoring required for fixed-duration regimens poorly and face higher infection risks with frequent hospital visits. Pirtobrutinib’s once-daily oral profile and low toxicity offer a safer, more manageable long-term option.