Cutaneous T-cell lymphoma (CTCL) is a heterogeneous malignant lymphoma originating from the skin and evolving from T-cells. It accounts for approximately 4% of non-Hodgkin lymphoma cases, classifying it as a rare disease. Common subtypes include Mycosis Fungoides (MF) and Sézary Syndrome (SS). In advanced stages, patients may experience involvement of lymph nodes, viscera, and blood, which significantly increases treatment difficulty. In recent years, with the approval of new drugs such as histone deacetylase (HDAC) inhibitors, brentuximab vedotin, and mogamulizumab, systemic treatment options have continuously evolved; however, they have not significantly improved patients’ overall survival. Therefore, further exploration of new drugs is needed to meet the treatment demands of CTCL patients.
At the American Society of Clinical Oncology (ASCO) Annual Meeting, held grandly in Chicago from May 30 to June 3, 2025, an oral presentation from China (Abstract No.: 2514) offered a new treatment direction for these patients: ICP-B05, an anti-CCR8 monoclonal antibody independently developed by Tianneng Jiancheng, showed promising therapeutic prospects in patients with relapsed/refractory (R/R) CTCL.
CCR8 is a chemokine receptor primarily expressed on the surface of tumor-infiltrating regulatory T cells (Treg). It forms a “protective shield” by recruiting immunosuppressive cells, hindering the body’s attack on tumors. Unlike traditional CCR4 monoclonal antibodies that only act on peripheral blood Tregs, ICP-B05 precisely recognizes CCR8-highly expressed Tregs within tumor tissues, achieving “precision strike.” This differentiated mechanism of action allows ICP-B05 to clear tumor-associated Tregs while maximally preserving normal immune function.
This study was led by Professor Zhiming Li from Sun Yat-sen University Cancer Center and employed a 3+3 design, with dose cohorts of 150 mg, 300 mg, 450 mg, and 600 mg (intravenous injection every two weeks). R/R CTCL patients (Mycosis Fungoides, Sézary Syndrome, Lymphomatoid Papulosis, Primary Cutaneous Anaplastic Large Cell Lymphoma) who had failed at least one standard systemic treatment were enrolled.
Interim data from 13 enrolled patients showed that ICP-B05 for R/R CTCL demonstrated:
01 Controllable Safety
Treatment-related adverse events (TRAEs) occurred in 12/13 (92.3%) patients. Grade ≥3 TRAEs occurred in 6/13 (46.2%) patients. The most common TRAEs were hematological events. Two patients (15.4%) reported serious AE (SAE). Infections were rare, and no fatal AE was reported.
02 Good Efficacy
In the skin lesion assessment (see Figure 1), 5 patients achieved partial response (PR), and 6 patients were evaluated as having stable disease (SD). Overall, 2 patients achieved PR. The median progression-free survival (PFS) was 11.4 months, with a 6-month PFS rate of 72.9% (the median PFS for mogamulizumab, a breakthrough therapeutic drug in this field, is 7.6 months ).
CCR8+ Biomarker Exploration: Among the 5 patients with high baseline CCR8 expression (≥10%), 4 (80%) achieved PR in skin efficacy assessment, while 1 patient with low expression also achieved skin PR, suggesting that high CCR8 expression may be a predictive biomarker for efficacy.
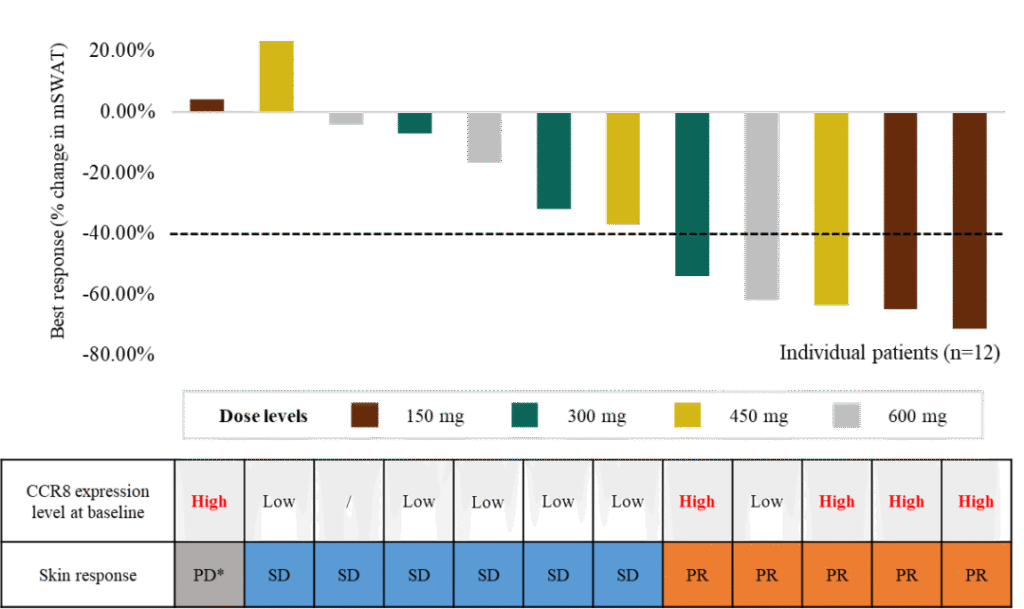
Figure 1. Skin Lesion Assessment and CCR8+ Biomarker
Concurrently, pharmacodynamic biomarkers (see Figure 2) showed that ICP-B05 treatment significantly cleared CCR8+ cells in tumor tissues and peripheral blood, with minimal impact on normal phenotypic Treg cells. This not only corroborated the favorable clinical efficacy but also explained the source of good safety, stemming from the sparing of the normal immune regulatory system.
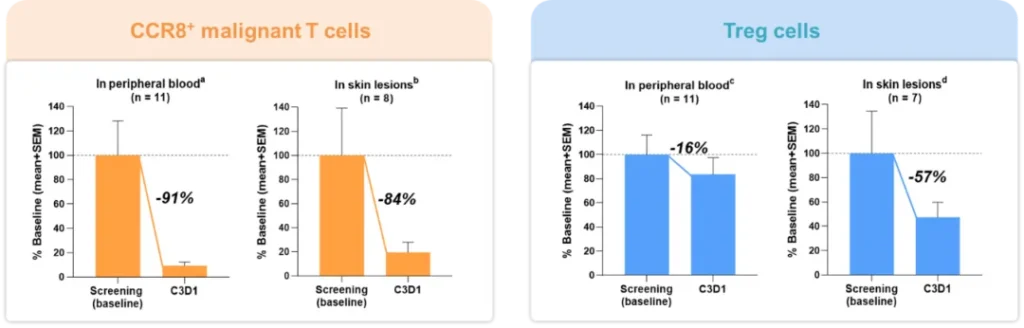
Figure 2. Pharmacodynamic Biomarkers
For R/R CTCL patients, ICP-B05 offers a new treatment option that can prolong PFS and improve quality of life. Although further validation is needed, its achievements have injected new momentum into CTCL clinical practice, marking a crucial step towards personalized immunotherapy in this field, and is expected to provide safer and more effective treatment options for patients in the future.
Literature Sources:
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023 Jan;98(1):193-209.
- Zhiming Li, et al. 2025ASCO Oral2514.
- Wen Y, Xia Y, Yang X, Li H, Gao Q. CCR8: a promising therapeutic target against tumor-infiltrating regulatory T cells. Trends Immunol. 2025 Feb;46(2):153-165.
- Kim YH, et al. Lancet Oncol. 2018 Sep;19(9):1192-1204.


