
In January 2024, a groundbreaking study spearheaded by Professor Gang An from Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, made its debut in the esteemed journal Haematologica (IF=11.047). The research, titled “MAF translocation remains a strong prognostic factor despite concurrent chromosomal abnormalities” marks a significant advancement in the understanding of multiple myeloma (MM). This comprehensive investigation meticulously analyzes the prognostic impact of MAF translocations, t(14;16) and t(14;20), within the context of MM, shedding light on their independent prognostic significance despite the presence of other chromosomal anomalies. This study not only enhances the scientific community’s comprehension of MM’s genetic complexity but also paves the way for refining risk stratification and tailoring treatment approaches for affected patients, thereby contributing to the ongoing efforts to improve outcomes in this challenging hematological malignancy.
Our retrospective analysis encompasses 830 patients newly diagnosed with MM. Among these, 34 were identified with the t(14;16) translocation, 4 with t(14;20), and 792 did not harbor either abnormality. The demographic and clinical characteristics of these patients were meticulously recorded, including age, gender, stage of disease according to the International Staging System (ISS), and treatment regimens initiated. Immunophenotypic analysis was conducted using the Cytomics FC 500 and FACSCanto flow cytometers, coupled with the CellQUEST program, to characterize the plasma cell population in detail.
An intriguing aspect of our study was the association of t(14;16)/t(14;20) with other high-risk chromosomal abnormalities, such as gain/amp(1q21), del(17p), and del(1p32). Notably, mutations in TP53 and TP53 bi-allelic inactivation were disproportionately represented in the cohort positive for these translocations. This finding suggests a complex genetic landscape in high-risk MM, where multiple concurrent aberrations may drive an aggressive clinical course.
The immunophenotypic analysis revealed a markedly lower expression of CD56 in the t(14;16)/t(14;20)-positive group compared to the negative cohort. This observation is particularly relevant as CD56, a neural cell adhesion molecule, has been implicated in the homing and adhesion of myeloma cells to the bone marrow microenvironment. Its reduced expression in the context of MAF translocations could signify a biologically distinct and more aggressive form of MM.
The core of our findings revolves around the prognostic implications of harboring t(14;16)/t(14;20). Patients with these translocations exhibited significantly worse progression-free survival (PFS) and overall survival (OS) compared to those without. The disparity persisted even after adjusting for potential confounders through propensity score matching (PSM), reinforcing the notion that these genetic abnormalities independently predict a poorer prognosis.
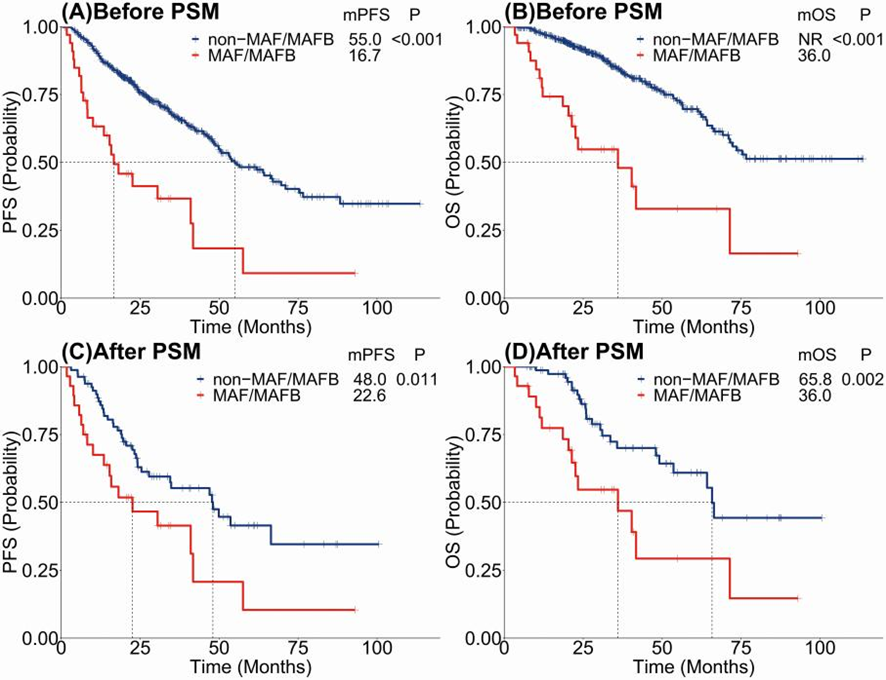
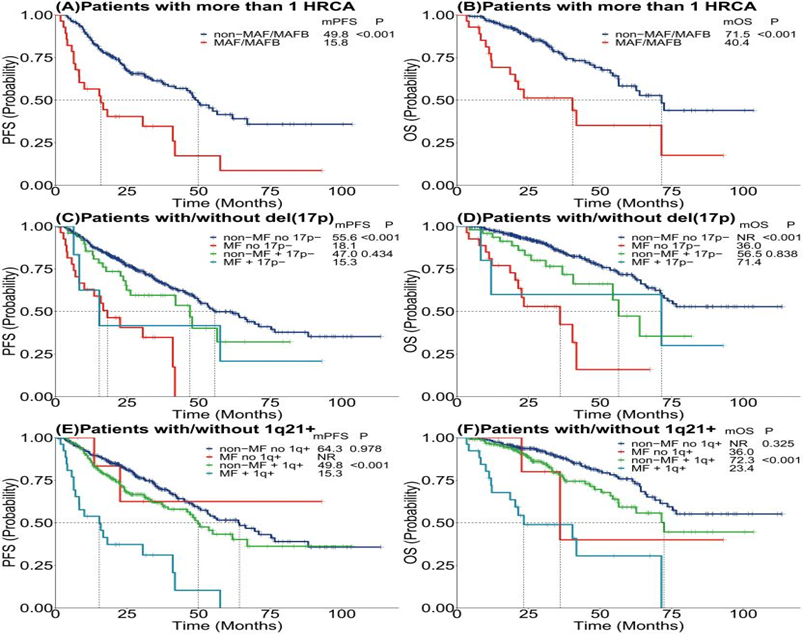
The identification of t(14;16) and t(14;20) as independent adverse prognostic factors in MM adds a crucial layer to the stratification of risk in this heterogeneous disease. These findings hold significant implications for the clinical management of MM, suggesting that patients with these translocations may benefit from more aggressive or targeted therapeutic approaches. Furthermore, our study underscores the necessity of incorporating comprehensive genetic screening into the diagnostic and monitoring processes for MM, enabling more personalized treatment strategies.
While our study sheds light on the importance of MAF translocations in MM, it also opens avenues for further research. Prospective studies are needed to explore the efficacy of targeted therapies in this specific subset of patients. Additionally, the molecular mechanisms underlying the aggressive behavior of MM cells harboring these translocations warrant deeper investigation. Understanding these pathways may reveal new therapeutic targets and strategies to overcome the adverse prognosis associated with these genetic abnormalities.
The study underscores the adverse prognostic significance of t(14;16) and t(14;20) translocations in multiple myeloma, highlighting their potential role as markers for aggressive disease and poor clinical outcomes. By identifying patients at high risk, this research contributes to the ongoing efforts to tailor treatment approaches and improve the prognosis for MM patients.


