Editor’s note :
Primary liver cancer is currently the 4th most common malignant tumor and the 2nd leading cause of tumor-related death in China. 75%~85% of these cases are Hepatocellular carcinoma (HCC), posing a serious threat to the health and lives of Chinese citizens. In recent years, with the progress in traditional therapies like surgery, intervention, radiation, targeted therapy, and immunotherapy, there are now more options available for liver cancer patients. Recent clinical studies have shown that the combined treatment plan of atezolizumab and bevacizumab (referred to as “T+A”) can reduce the mortality risk of advanced liver cancer patients by 42% compared to the traditional targeted drug sorafenib, setting a new standard for the first-line treatment of advanced liver cancer. However, the overall survival rate of liver cancer patients remains unsatisfactory, with a 5-year survival rate of about 20%. Due to the heterogeneity of liver cancer, there is still a significant variance in the treatment response and prognosis of patients with the same clinical stage. How to select the most suitable individualized treatment method for different liver cancer patients, to maximize therapeutic effects, is a pressing issue in the clinic.In this context, Dr. Decai Yu’s team at the Hepatobiliary and Liver Transplant Surgery Department of Nanjing Drum Tower Hospital, Nanjing Medical University, China, has developed a molecular typing indicator for liver cancer based on fatty acid degradation (FAD) metabolism. This aims to enhance precision diagnosis and treatment of liver cancer, and thereby improve patient survival rates. At the recently concluded 17th Annual Meeting of the International Liver Cancer Association (ILCA 2023), Dr. Binghua Li from Dr Yu’s team presented this research in a poster (conference abstract number: P-04), which was selected as one of the top 10 posters of the conference.
Molecular typing of tumors refers to classifying tumors through molecular analysis, transitioning tumor classification from traditional morphology-based methods to molecular characteristic-based ones. This molecular pathology-based classification reflects deeper features of tumors, providing support for diagnosis, treatment, and prognosis prediction, thus supplementing the limitations of the clinical staging system. In response to the clinical challenges mentioned above, Professor Yu’s team conducted in-depth research using multi-omics methods.
The study collected samples from 41 liver cancer patients, including cancer tissues, adjacent non-cancer tissues, and peripheral serum (17 of whom underwent anti-PD1 treatment). These samples underwent transcriptome sequencing (n=66), lipidomics (n=63), metabolomics (n=41), proteomics (n=41), and PCR chip detection (n=41), followed by prognosis follow-up. For those who received PD1 treatment, therapeutic efficacy was evaluated radiologically according to the RECIST 1.1 criteria.
Additionally, the research collated nearly 60 public datasets of liver cancer and other tumors, encompassing gene chips, bulk RNA-seq, proteomics, single-cell sequencing (scRNA-seq), metabolomics, and more. The total sample size approached 8,000, including human liver cancer tissues, cell lines, mouse liver cancer models, and other tumor types. The data underwent comprehensive analysis. The team also collaborated with pharmaceutical companies to develop 104 patient-derived xenograft (PDX) models from human liver cancer tissues and tested the sensitivity of 24 of these models to sorafenib.
The study discovered that liver cancer patients can be categorized into three types, F1, F2, and F3, based on FAD activity. Different subtypes of liver cancer have varying clinical pathology, genomic features, epigenetic regulation, metabolism, signaling pathways, and immune characteristics. Importantly, the study proposes new precision treatment strategies for liver cancer based on FAD molecular typing. F1 liver cancer is sensitive to sorafenib while F3 is resistant. F1 liver cancer is resistant to TACE treatment, whereas F3 is sensitive. F1 is sensitive to PD1 treatment, whereas F2 and F3 are resistant. F1 is sensitive to PD-L1 treatment, while F3 is resistant. F1 and F2 are sensitive to the T+A treatment, but F3 is resistant.
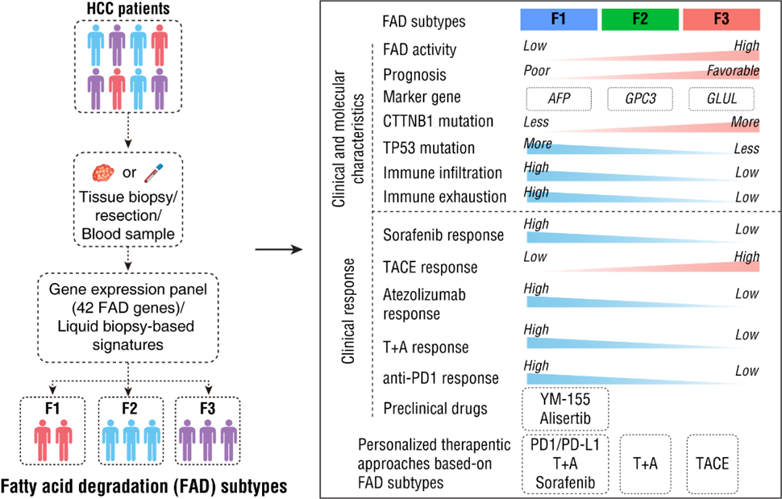
The study suggests that liquid biopsy methods can be used to diagnose FAD typing by detecting serum metabolites or proteins, allowing non-invasive prediction of therapeutic outcomes in liver cancer patients. The findings hold promise for informing treatment selection for advanced liver cancer patients. The team is currently conducting multiple multicenter prospective clinical studies to validate the application of FAD typing in precision liver cancer treatment.
The results of this study were published in the top hepatology journal, Hepatology, on August 7, 2023.
Reflections on Attending the Conference
Dr. Binghua Li: The ILCA conference is an international event for liver cancer research. I am delighted and honored to have had the opportunity to share our team’s latest findings at the ILCA conference. I met many other teams conducting excellent liver cancer multi-omics/typing research and had in-depth exchanges. We are currently undertaking numerous multicenter prospective clinical studies, verifying the role of FAD typing in the selection and efficacy prediction of liver cancer treatment. I hope to have the opportunity to share further research progress at the next ILCA conference.


