As HIV treatment enters the post-ART era, drug side effects and complications have become significant factors affecting the quality of life for patients. Approximately 20% of patients, after 1-2 years of treatment, have viral loads below the detectable limit, but their CD4 cell count remains below normal levels, leading to poorer clinical outcomes.
From 2021 to 2022, the Shanghai Public Health Clinical Center conducted a clinical study on the use of thymosin α1 for the treatment of HIV immunological non-responders. The study results were presented as an e-poster at the IAS 2023 conference (Abstract No. 4511).
A total of 20 participants were enrolled in the study, undergoing treatment and follow-up for a duration of six months (Registration No. NCT04963712).
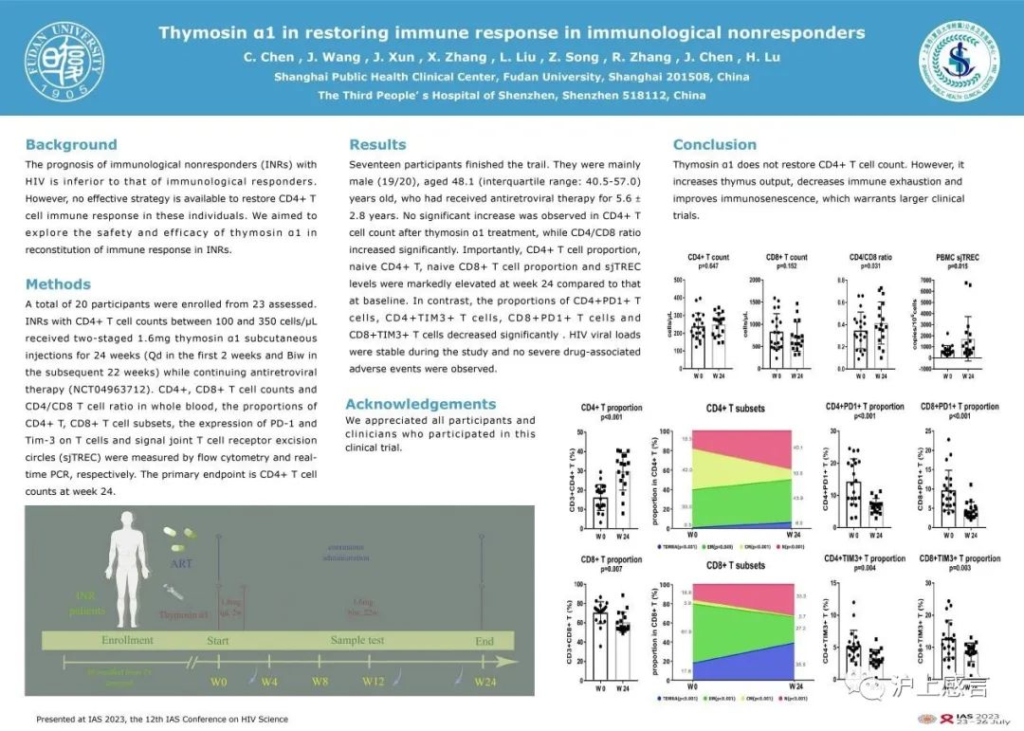
At the end of the trial, there was no significant increase in the CD4 cell count of the participants (239.2 cells/µL vs. 250.2 cells/µL, P = 0.647). However, there was a slight increase in the CD4/CD8 ratio (0.35 vs. 0.42, P = 0.031).
Furthermore, the proportion of the participants’ CD4T cells significantly increased (16.2% vs. 29.8%, P<0.001), as did the proportion of naive CD4T cells (18.5% vs. 40.1%, P<0.001). There was also a notable increase in the thymic output marker PBMC sjTREC (661.2 copies/10^6 cells vs. 1724.5 copies/10^6 cells, P = 0.015). Simultaneously, the T-cell exhaustion markers PD-1 and TIM-3 showed significant reductions in both CD4T and CD8T cells (P <0.01 for both). Moreover, no apparent adverse reactions were reported among the participants during the treatment, and the viral load remained stable.
In conclusion, thymosin α1 demonstrates good safety, but it does not significantly increase the CD4 count in HIV immunological non-responder patients. Additionally, thymosin α1 effectively enhances the CD4/CD8 ratio, increases the proportion of CD4T cells, especially the naive cells, reduces the degree of T-cell immune exhaustion, and boosts thymic output levels.


