Editor’s Note:
The 2023 European Society for Medical Oncology (ESMO) Annual Meeting took place from October 20th to 24th in Madrid, Spain. The Oncology Insights Reporting Team delved deep into the front lines to witness international advancements and to witness the progress of China’s anti-tumor efforts on the global stage. Professor Huiyan Luo from Sun Yat-sen University Cancer Center presented a crucial oral presentation (Abstract ID: 1026MO) at this conference. In this on-site interview, Professor Huiyan Luo shared insights into the study of the treatment of advanced solid tumors using the dual-target antibody SHR-1701, which targets PD-L1 and TGF-βRII, in combination with the VEGF inhibitor bevacizumab, as well as her thoughts on future directions for the treatment of advanced tumors. This article compiles the relevant content for readers.

Professor Huiyan Luo
Director of Internal Medicine, Sun Yat-sen University Cancer Center
Doctoral Supervisor
Deputy Chair of the Ethics Committee, Sun Yat-sen University Cancer Center
Visiting Scholar at the University of California, San Diego, Department of Medical Genetics
Recipient of the 2019 USCACA-AFCR Scholarship Award
Recipient of the 2021 “People’s Good Doctor Golden Camellia Program” for Outstanding Contributions in the Field of Gastric Cancer
Vice-Chair of the Young Committee of the Chinese Anti-Cancer Association Gastric Cancer Professional Committee
Chair of the Guangdong Clinical Medical Society Digestive Tumor Comprehensive Treatment Youth Committee
Vice-Chair of the Guangdong Medical Education Association Oncology Professional Committee
Vice-Chair of the Guangdong Precision Medicine Application Association Tumor Comprehensive Treatment Branch
Vice-Chair of the Guangdong Clinical Medical Society Esophageal Tumor Special Committee
Author of over 70 articles in domestic and international journals, with first/co-first author publications in important journals such as JAMA, Nature Materials, Lancet Oncology, Science Translational Medicine, Annals of Oncology, and others.
Oncology Frontier : This year at the ESMO conference, you presented your study on the Phase Ib/II trial of SHR-1701 in combination with bevacizumab for the treatment of advanced solid tumors, avoiding chemotherapy. Could you please explain the initial motivation behind this research?
Professor Huiyan Luo
Bevacizumab is a humanized anti-VEGF monoclonal antibody widely used in the treatment of various cancers to inhibit angiogenesis. It is indicated for metastatic colorectal cancer, non-small cell lung cancer, malignant glioma, metastatic renal cell carcinoma, and metastatic cervical cancer, among others. Studies have shown that simultaneously inhibiting VEGF and TGF-β can reverse the tumor immune microenvironment, enhance immunotherapy sensitivity, and improve the effectiveness of immunotherapy.
SHR-1701 injection is a dual-function fusion protein developed independently by Hengrui Medicine with intellectual property rights. It can simultaneously inhibit the PD-L1 and TGF-βRII targets, activate effector T cells, and improve the immunomodulatory effects in the tumor microenvironment. Ultimately, it effectively promotes the immune system’s ability to target tumor cells.
While immune checkpoint inhibitors, such as anti-PD-1/PD-L1 antibodies, have achieved unprecedented success in multiple cancer types, it’s essential to acknowledge that a significant proportion of patients remain insensitive to this therapy. Currently, combination therapy is expected to be one of the future directions of cancer immunotherapy. The challenge lies in determining how to combine these therapies effectively. Many studies have already explored the combination of PD-1/PD-L1 inhibitors with other immune checkpoint inhibitors, traditional chemotherapy drugs, or targeted therapies. The results of these studies have shown that combining with anti-angiogenesis drugs can significantly enhance the efficacy of anti-PD-1/PD-L1 treatment across various cancers, with manageable safety profiles. Therefore, we initiated this study to investigate the effectiveness and safety of SHR-1701, a dual-function fusion protein targeting PD-L1 and TGF-βRII, in combination with the VEGF inhibitor bevacizumab for the treatment of advanced solid tumors, with the hope of providing a more effective treatment for patients with advanced tumors.
Oncology Frontier: Could you please provide an overview of the study’s design and discuss any issues or challenges encountered during the research process?
Professor Huiyan Luo
This study consisted of two phases: Phase Ib, primarily focused on exploring drug dosages and safety, and Phase II, which aimed to expand the sample size and explore efficacy. The key focus of the Phase Ib study was to determine the Recommended Phase II Dose (RP2D). Phase II was divided into three cohorts: Cohort 1 included treatment-naive advanced gastric and gastroesophageal junction cancer (GC/GEJC) patients with PD-L1 CPS ≥1 and HER2 negativity; Cohort 2 included patients with advanced GC/GEJC who had received up to two prior systemic therapies; Cohort 3 included patients with non-squamous non-small cell lung cancer (nsqNSCLC) who were driver gene-negative and had failed platinum-based chemotherapy and immunotherapy. The primary endpoint of the Phase II study was the objective response rate (ORR). We aimed to verify whether dual inhibition of VEGF and TGF-ß could reverse the tumor immune microenvironment, enhance immunotherapy sensitivity, and benefit patients with advanced tumors.
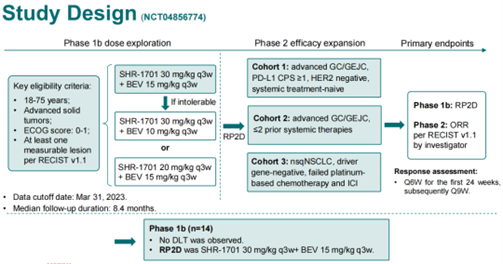
As for the challenges we encountered, there were two main areas. First, there was concern about the risk of bleeding in patients. In the initial design of the study, we were somewhat concerned about the dosages for the combination of SHR-1701 and bevacizumab because bevacizumab can inhibit angiogenesis, which may increase the risk of bleeding. Some earlier studies had shown that TGF-ß inhibitors could also lead to decreased platelet counts, increasing the risk of bleeding. Therefore, the combination of these two drugs might further increase the risk of bleeding. The second challenge was the selection of the patient population that might benefit from SHR-1701 in combination with bevacizumab. To address this, we referred to the data from the earlier SHR-1701 monotherapy study, which informed the design of the study with three different cohorts. Looking at the results of the study so far, it has been very successful.
Oncology Frontier : Could you please provide an overview of the study’s results, including efficacy and safety data, and explain why patients with PD-L1 CPS ≥5 in Cohort 2 of gastric/gastroesophageal junction cancer achieved significantly better median PFS than other patients?
Professor Huiyan Luo
To begin with, I’d like to share the study results. In the Phase Ib part of the study, which focused on determining the recommended phase II dose (RP2D) and safety, 14 patients were enrolled, and the treatment showed good safety outcomes. No dose-limiting toxicity (DLT) was observed, and the final RP2D was determined to be bevacizumab at 15 mg/kg and SHR-1701 at 30 mg/kg, administered once every three weeks.
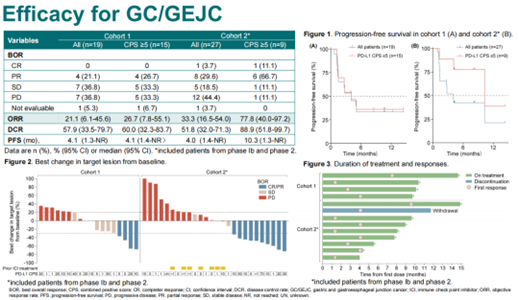
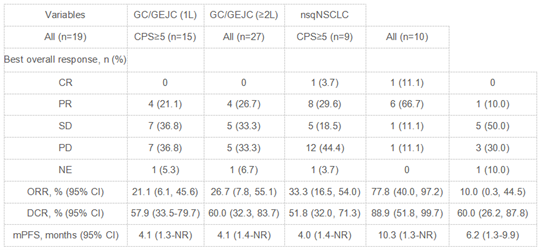
In the Phase II stage, Cohort 1 included 19 patients with advanced gastric and gastroesophageal junction cancer who were PD-L1 CPS ≥1, HER2 negative, and were treatment-naive. The objective response rate (ORR) in this cohort was 21.1%, with a median progression-free survival (mPFS) of 4.1 months. Cohort 3 consisted of 10 patients with non-squamous non-small cell lung cancer (nsqNSCLC) who were driver gene-negative and had previously failed platinum-based chemotherapy and immunotherapy. The ORR in this cohort was 10%, with an mPFS of 6.2 months. Cohort 2 included 27 patients with previously treated advanced gastric/gastroesophageal junction cancer, which included patients from the Phase Ib study. The overall ORR in Cohort 2 was 33.3%, with an mPFS of 4.0 months. Notably, in Cohort 2, patients with a PD-L1 CPS ≥5 had an ORR of 77.8% and an mPFS of 10.3 months, which was significantly better than other patients.
The reason for the significantly better mPFS in patients with PD-L1 CPS ≥5 in Cohort 2 may be attributed to several factors. First, these patients had already achieved better outcomes during first-line immunotherapy. Second, in Cohort 1, approximately 30% of patients had undergone radical gastrectomy, whereas in Cohort 2, nearly 70% of patients had undergone radical gastrectomy. The majority of patients in Cohort 1 experienced progression at the primary site, and the resection of the primary tumor might have contributed to the better outcomes in Cohort 2. Lastly, patients in Cohort 2 had relatively lower tumor burdens, making them more likely to benefit from the “chemo-free” approach of SHR-1701 in combination with bevacizumab. However, it’s important to note that because this study is a small sample Phase II trial, there might be biases due to the limited sample size. Nevertheless, the favorable efficacy data from the Phase II study have bolstered our confidence in conducting a larger Phase III study to further explore the combination of SHR-1701 with bevacizumab for the treatment of advanced solid tumors.
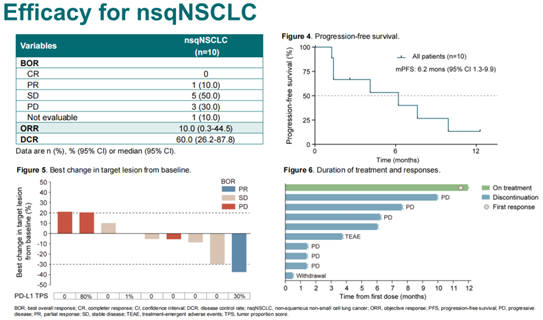
Oncology Frontier : The study also showed promising results in previously treated NSCLC patients who had failed immunotherapy. Could you share the clinical implications of these findings for immunotherapy-refractory patients?
Professor Huiyan Luo
In the Phase II study, 10 patients with non-squamous non-small cell lung cancer (nsqNSCLC) were enrolled, all of whom had failed platinum-based chemotherapy and immunotherapy, and were negative for driver genes. One patient in this cohort achieved a partial response (PR) and had a disease control period of over 12 months. This suggests that patients who have developed resistance to immunotherapy may also benefit from treatment with SHR-1701 in combination with bevacizumab. We need to further explore the patient populations that would benefit from SHR-1701 in combination with bevacizumab as a form of immunotherapy cross-line treatment.
Abstract
1026MO – A phase Ib/II study of SHR-1701 (a bifunctional anti-PD-L1/TGF-βRII agent) in combination with bevacizumab (BEV) in patients with advanced solid tumors
Background
Accumulative evidence demonstrates that anti-angiogenesis plus immune checkpoint inhibitor (ICI) has a synergistic antitumor effect in various types of tumors. Thus, we assessed the safety and efficacy of SHR-1701 plus BEV in patients (pts) with advanced solid tumors.
Methods
This is a multicenter, open-label, phase 1b/2 study. The primary objective of phase 1b part was to determine the recommended phase 2 dose (RP2D) in pts with advanced solid tumors. Phase 2 part enrolled: 1) pts with advanced gastric and gastroesophageal junction cancer (GC/GEJC), PD-L1 CPS ≥1, HER2 negative, who had not received prior systemic therapy (1L); 2) pts with advanced GC/GEJC who had received ≤2 prior systemic therapies; 3) pts with recurrent or metastatic non-squamous non-small cell lung cancer (nsqNSCLC) who had failed ≤2 lines of systemic treatment (including platinum-based chemotherapy and ICI), driver gene-negative. SHR-1701 and BEV were given once every 3-week cycle. Primary endpoint of phase 2 part was objective response rate (ORR).
Results
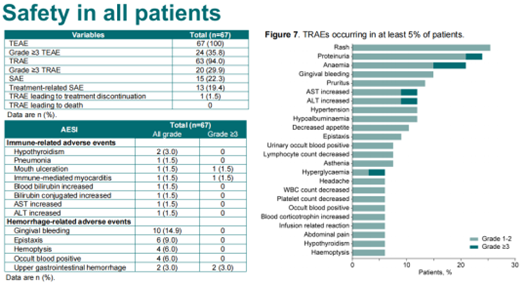
As of Mar 31, 2023, a total of 67 pts with advanced solid tumor were enrolled, including 19 systemic treatment-naive GC/GEJC pts, 27 pre-treated GC/GEJC pts, and 10 nsqNSCLC pts. No DLT was observed in phase 1b part. The RP2D was determined to be SHR-1701 30 mg/kg plus BEV 15 mg/kg, Q3W. With a median follow-up of 8.4 mos, the ORR was 21.1%, 33.3%, and 10.0% in the three cohorts, respectively (Table). Treatment-related adverse events (TRAEs) occurred in 94.0% of pts. Grade 3 TRAEs were reported in 29.4% of pts, with the most common ones being anemia (5.8%), increased ALT (2.9%), and increased AST (2.9%). No grade 4/5 TRAEs were reported. Table: 1026MO
Efficacy outcomes
Conclusions
SHR-1701 plus BEV showed encouraging antitumor activity with a favorable safety profile in pts with advanced GC/GEJC and nsqNSCLC.
Clinical trial identification
NCT04856774.


