Editor’s Note:
Anti-HER2 therapy has traditionally been implemented based on HER2 expression detected through immunohistochemistry. In clinical practice, a small subset of patients with HER2-negative expression may have HER2 gene mutations (HER2m) but lack corresponding standard treatment options. At the 2023 European Society for Medical Oncology (ESMO) annual meeting, the latest DESTINY-PanTumor01 study was unveiled, breaking the silence and revealing the therapeutic potential of trastuzumab deruxtecan (T-DXd), an anti-HER2 antibody-drug conjugate (ADC), in various HER2-negative tumors with HER2 mutations, thus expanding the landscape of T-DXd anti-HER2 treatment.
Overview of HER2 Mutations and Unmet Clinical Needs
Human epidermal growth factor receptor 2 (HER2) gene mutations (HER2m) are present in various cancers, including breast cancer, lung cancer, and gynecological tumors, with a positive rate of no more than 5%[1]. For example, in breast cancer, the rate is approximately 3%[2], in lung cancer, it ranges from 2% to 4%[3], and in gastric cancer, it’s about 1.1%[4]. HER2 gene mutations typically occur in tumors that are negative for HER2 overexpression or amplification[5]. HER2 mutations often occur in the intracellular kinase domain, such as 20-exon insertion mutations, or they can occur in the transmembrane and extracellular regions. Previous studies have shown that HER2 mutations can lead to increased kinase activity, receptor homodimerization, changes in protein conformation or function, abnormal activation of downstream signaling pathways, and promotion of tumor proliferation, activation, invasion, or metastasis. In short, HER2 mutations can drive tumor development[6,7].
Although the role of HER2 mutations in cancer has been confirmed, and there have been attempts to treat this population with corresponding drugs, the overall efficacy has been limited. As a result, despite more than ten anti-HER2 drugs on the market, there has been no approved targeted therapy for HER2-mutant tumors. With the development and progress of new drugs, the novel ADC drug T-DXd has demonstrated unprecedented efficacy in HER2-positive breast cancer and its approval has continued to expand, covering HER2-positive gastric cancer and HER2-low-expressing breast cancer. In 2022, it became the first approved anti-HER2 targeted drug for HER2-mutant non-small cell lung cancer (NSCLC).
The DESTINY-PanTumor02 study presented at the 2023 American Society of Clinical Oncology (ASCO) annual meeting[8] investigated the potential of T-DXd for HER2-expressing solid tumors. Building on this, the DESTINY-PanTumor01 study presented at ESMO this year further explored its effectiveness in HER2-mutant solid tumors, attempting to expand the anti-HER2 treatment landscape to include HER2-mutant populations.
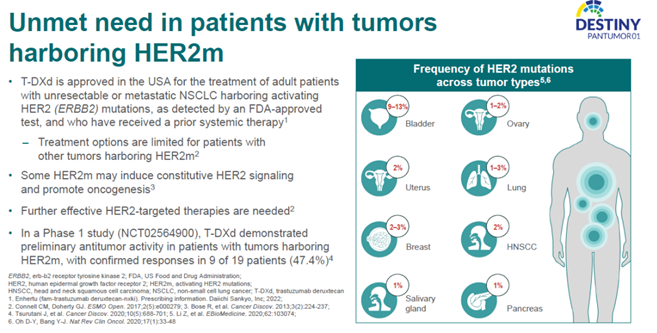
Unmet Needs in Patients with HER2m Tumors
DESTINY-PanTumor01: A Groundbreaking Exploration of HER2m in the Anti-HER2 Treatment Landscape
The phase II DESTINY-PanTumor01 study primarily explored the feasibility of using T-DXd in patients with HER2m solid tumors. The main inclusion criteria for the study were unresectable and/or metastatic HER2m solid tumor patients, those without suitable alternative options after prior treatment, and patients who had previously received anti-HER2 treatment. The main exclusion criteria included HER2-positive (IHC 3+ or IHC 2+/ISH+) breast cancer, gastric cancer, gastroesophageal junction cancer, HER2m NSCLC, and patients with a history of chronic obstructive pulmonary disease (COPD). Patients received T-DXd treatment at a dose of 5.4 mg/kg every 3 weeks, and the primary endpoint was the confirmed objective response rate (ORR) as assessed by an independent review committee (ICR). Each tumor type included no more than 20 patients.
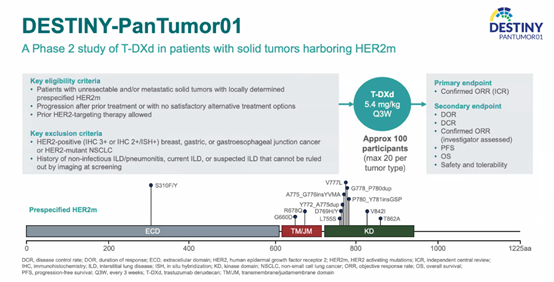
Study Design
A total of 102 patients with solid tumors, including breast cancer, colorectal cancer, and bile duct cancer, participated in the study and were included in the analysis. The median age was 66.5 years (ranging from 36 to 84 years), and the median number of prior treatment lines was 3 (ranging from 1 to 13). 17.6% of the patients had received anti-HER2 treatment, 9.8% of patients were HER2 IHC3+ as determined by central evaluation, and 36.3% of patients had IHC 0 expression. Among these, 51.0% had kinase domain HER2m, 33.3% had extracellular domain HER2m, and 16.7% had transmembrane domain/membrane-proximal structure HER2m.
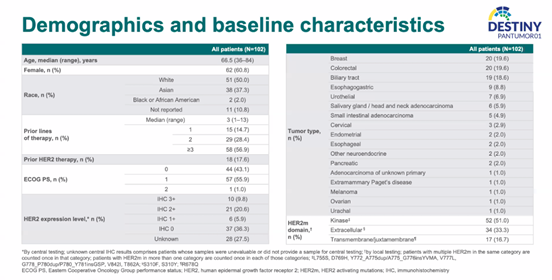
Patient Baseline Characteristics
The median number of treatment cycles with T-DXd was 5.0 (ranging from 1 to 29 cycles), and the median follow-up was 8.6 months. The results showed that the ICR-confirmed ORR for the overall population was 29.4%. Among different cancer types, those with prominent objective responses included breast cancer with 10 cases (n=20), colorectal cancer with 4 cases (n=20), urothelial carcinoma with 2 cases (n=7), head and neck tumors with 4 cases (n=6), gynecological tumors with 4 cases (n=6), and other tumors with 3 cases (n=10). It is worth noting that among patients with objective responses, four cases of breast cancer were HER2 IHC 0, and there was one case each of bile duct cancer, esophageal cancer, and head and neck tumors with HER2 IHC 0.
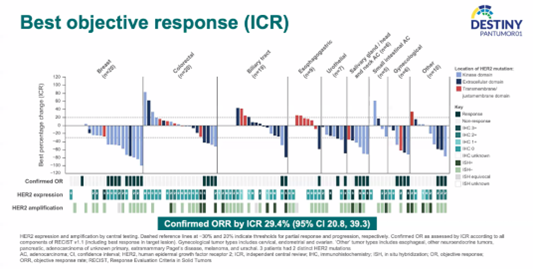
ICR-Confirmed ORR
The study also highlighted the duration of response (DOR): among patients with confirmed ORR, the median DOR has not yet been reached, with 54.2% of patients experiencing sustained remission for more than 18 months.
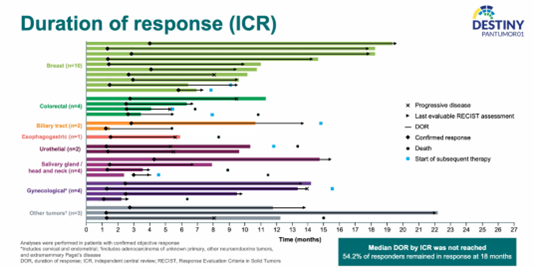
DOR in Patients with Confirmed ORR
For the overall population, the ICR-assessed progression-free survival (PFS) was 5.4 months, and the median overall survival (mOS) was 10.9 months.
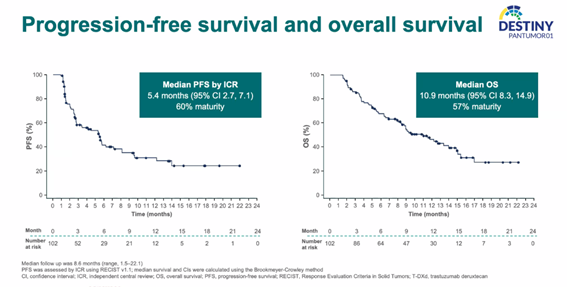
PFS and OS
In terms of safety, T-DXd did not reveal any new adverse event signals. The primary 3-grade or higher adverse events were neutropenia (6.9%), anemia (4.9%), and fatigue (2.9%). The rate of grade 3 or higher interstitial lung disease (ILD) was 3%, consistent with previous studies.
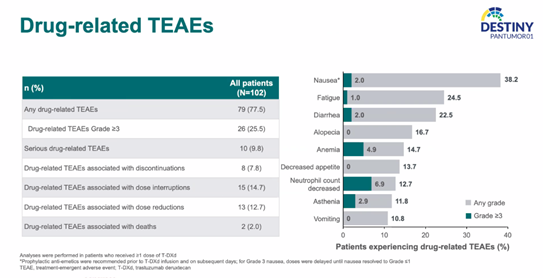
Safety
Overall, in this study, T-DXd demonstrated favorable efficacy and safety in patients with HER2m solid tumors such as breast cancer and colorectal cancer, offering potential treatment options for such patients. Notably, breast cancer achieved an objective response rate of 50%. Although specific DOR data for individual tumor types were not provided, the duration of
treatment in the form of a survival curve indicated that breast cancer had a promising DOR, making it a highly exploratory tumor type.
Expert Commentary
In the ever-evolving landscape of cancer, despite the low prevalence of HER2m, the progress in genetic screening and new drug development has placed an increasing emphasis on the precise treatment of HER2m. In the field of lung cancer, HER2m testing has become a recommended category when EGFR, ALK, and ROS1 are negative, and next-generation sequencing (NGS) is strongly recommended by experts in China and can be used to recommend corresponding diagnostic and treatment methods[4]. This has also set the direction for the detection and treatment of HER2m in solid tumors like breast cancer.
Focusing on breast cancer, the HER2m positive rate is around 3%, with a higher prevalence in HER2-negative breast cancer, which is associated with a poorer prognosis. In breast cancer, HER2 mutations are mainly point mutations, with common sites including L755S (86%), V777L (49%), D769H (28%), and S310F (20%)[3]. There are also some cases of co-mutations. Previous studies have shown that in HER2-negative breast cancer, the overall proportion of HER2 variations is 2.88% (17/589), including HER2 amplification, point mutations, and gene fusions[10]. It has been observed that several cases of HER2 amplification were detected in HER2-negative patients, and a small proportion of HER2-positive patients had NGS results showing no amplification. This suggests that combining NGS with traditional molecular subtyping can provide more treatment-related information, including HER2 point mutations as mentioned in this study.
Due to the excellent efficacy of anti-HER2 therapy in HER2-positive breast cancer, efforts have been made to treat HER2m breast cancer. The SUMMIT study explored the efficacy and safety of neratinib as a single or combination therapy in various solid tumors with HER2 or HER3 mutations. In this study, in patients with HER2m breast cancer who had progressed after CDK4/6 inhibitor treatment, the combination of neratinib, fulvestrant, and trastuzumab (N + F + T) achieved an ORR of 39% and a median PFS of 8.3 months[11]. In another study, single-agent neratinib treatment for HER2m breast cancer (N=16) achieved a clinical benefit rate (CBR) of only 31% and a median PFS of 16 weeks[12]. A study in China showed that single-agent pyrotinib used for patients with HER2 mutations and HER2 amplification-negative patients (N=10) achieved an ORR of 40% and a median PFS of 4.9 months[13]. Currently, there is relatively limited clinical data for HER2m breast cancer, with tyrosine kinase inhibitors (TKIs) being the main focus. Apart from T-DXd, there have been no reports of the efficacy of other ADCs for HER2m breast cancer.
The DESTINY-PanTumor01 study presented at ESMO 2023 showcased the potential of the novel ADC drug T-DXd in HER2m breast cancer, with a 50% ORR and excellent duration of response, demonstrating its treatment potential in this population. Further updates are awaited from subsequent follow-up data.
Possible Mechanisms Behind T-DXd Efficacy
Previously, HER2 mutations were considered one of the mechanisms of resistance to anti-HER2 treatment in HER2-positive breast cancer, as they can lead to abnormal activation of HER2 kinase, resulting in resistance to monoclonal antibody drugs that target extracellular binding sites. Mutations in the kinase domain can also affect the binding of TKIs and lead to resistance. It has also been reported that HER2m breast cancer, initially sensitive to TKIs, can develop resistance due to secondary HER2 mutations.
ADC drugs not only inhibit the HER2 pathway but also deliver toxic payloads to tumor cells through antibodies, causing cytotoxic effects[15]. The high drug-to-antibody ratio and high activity of T-DXd may contribute to the improvement in ORR and DOR. This may also explain why some tumors with IHC 0 expression responded effectively. In addition, previous studies in NSCLC have reported that HER2 mutations can promote the internalization of T-DXd[16], which may also be a reason why IHC 0 patients responded effectively. However, it’s important to note that in breast cancer, IHC 0 indicates “no staining or ≤10% of infiltrating cancer cells show incomplete, weak cell membrane staining,” which does not necessarily mean there is no HER2 expression. This may also be one of the potential reasons.
T-DXd has overcome numerous challenges in breast cancer, from HER2-positive breast cancer to HER2-low-expressing breast cancer, and now to HER2-mutant breast cancer, becoming a treatment option for HER2-targeted tumors in breast cancer. The two DESTINY-PanTumor studies have targeted a wide range of tumors with HER2 expression/mutations, bringing T-DXd to a broader range of HER2-targeted tumors and providing a new choice for patients with HER2-targeted tumors.

Professor Ning Liao
Doctor of Medicine, Professor, Doctoral Supervisor
Administrative Director of the Department of Surgical Breast Cancer, Guangdong Provincial People’s Hospital
Director of the International Council of the American Society of Surgical Oncologists (SSO)
Director of the International Council of the International Sentinel Node Society (ISNS)
Member of the Expert Group for the Chinese Version of the National Comprehensive Cancer Network (NCCN) Breast Cancer Guidelines
Member of the Expert Group for the National Health and Health Commission’s “Guidelines for the Diagnosis and Treatment of Breast Cancer”
Member of the National Health and Health Commission’s Rational Drug Use Expert Committee “Oncology Drug Group”
References
[1] Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers [published correction appears in Nature. 2019 Feb;566(7745):E11-E12]. Nature. 2018;554(7691):189-194.
[2] Petrelli F, Tomasello G, Barni S, Lonati V, Passalacqua R, Ghidini M. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat. 2017;166(2):339-349.
[3] Zhang S, et al. Chinese expert consensus on the diagnosis and treatment of HER2-altered non-small cell lung cancer. Thorac Cancer. 2023 Jan;14(1):91-104
[4] Laura Schubert et al. Incidence of ERBB gene fusions (EGFR, ERBB2, ERBB4) across tumor types. 2021 ASCO. Abstract 3091.
[5] Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2(5):e000279. Published 2017 Nov 24. doi:10.1136/esmoopen-2017-000279
[6] Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224-237. doi:10.1158/2159-8290.CD-12-0349
[7] Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832-841. doi:10.1158/2159-8290.CD-14-1211
[8] Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results[J]. 2023 ASCO LBA3000
[9] Li BT, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXD) in patients (pts) with solid tumors harboring specific HER2-activating mutations (HER2m): primary results from the international phase 2 DESTINY-PanTumor01 (DPT-01) study. ESMO Congress 2023, Abstract 654O
[10] Xiao W, Zhang G, Chen B, et al. Characterization of Frequently Mutated Cancer Genes and Tumor Mutation Burden in Chinese Breast Cancer. Front Oncol. 2021;11:618767. Published 2021 Apr 21. doi:10.3389/fonc.2021.618767
[11] Jhaveri K, Eli LD, Wildiers H, et al. Neratinib + fulvestrant + trastuzumab for HR-positive, HER2-negative, HER2-mutant metastatic breast cancer: outcomes and biomarker analysis from the SUMMIT trial. Ann Oncol. 2023;34(10):885-898.
[12] Ma CX, Bose R, Gao F, et al. Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin Cancer Res. 2017;23(19):5687-5695.
[13] Yi Z, Rong G, Guan Y, et al. Molecular landscape and efficacy of HER2-targeted therapy in patients with HER2-mutated metastatic breast cancer. NPJ Breast Cancer. 2020;6:59. Published 2020 Oct 30.
[14] Marín A, Mamun AA, Patel H, et al. Acquired Secondary HER2 Mutations Enhance HER2/MAPK Signaling and Promote Resistance to HER2 Kinase Inhibition in Breast Cancer. Cancer Res. 2023;83(18):3145-3158.
[15] Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo). 2019;67(3):173-185.
[16] Li BT, Michelini F, Misale S, et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020;10(5):674-687.

