In September 2023, a study led by Professor Rong Fu from Tianjin Medical University General Hospital was published in the prestigious international academic journal —— American Journal of Hematology(IF=13.268). The title of the study is "Efficacy and safety of the C5 inhibitor crovalimab in complement inhibitor-naive patients with PNH (COMMODORE 3): A multicenter, Phase 3, single-arm study". The study introduce that crovalimab as a highly effective and well-tolerated therapeutic option for complement inhibitor-naive patients grappling with the challenges of PNH.
Paroxysmal Nocturnal Hemoglobinuria (PNH) is a rare and debilitating hematological disease caused by a genetic mutation in the PIG-A gene, resulting in deficient glycosylphosphatidylinositol (GPI) anchor synthesis. This deficiency affects glycosylphosphatidylinositol-linked membrane proteins, including crucial complement regulatory proteins CD55 and CD59. Normally, these proteins prevent complement-mediated lysis of red blood cells, which is responsible for the hallmark symptoms of PNH, including intravascular hemolysis, anemia, and thromboembolic events. Currently, the standard treatment for PNH involves complement inhibition with C5 inhibitors such as eculizumab and ravulizumab.
This study aimed to comprehensively evaluate the efficacy and safety of crovalimab, a novel C5 inhibitor, in complement inhibitor-naive patients with PNH, particularly in regions where access to standard complement inhibitors may be limited.
The Phase 3, open-label, multicenter, single-arm trial enrolled 51 complement inhibitor-naive patients with PNH from five medical centers in China. Eligible patients were aged ≥12 years, weighed ≥40 kg, had an LDH level of ≥2 times the upper limit of normal (ULN), and had received at least four transfusions of packed red blood cells (pRBCs) in the previous 12 months. Crovalimab was administered as loading doses (comprising one intravenous infusion followed by four subcutaneous injections) and subsequent subcutaneous maintenance doses administered every 4 weeks. The primary efficacy endpoints included evaluating the mean proportion of patients with hemolysis control (defined as LDH ≤1.5 times ULN) from Weeks 5 through 25 and assessing the difference in the proportion of patients achieving transfusion avoidance from baseline through Week 25 compared to the 24 weeks prior to screening. Secondary endpoints encompassed breakthrough hemolysis, stabilized hemoglobin levels, and fatigue improvement.
Primary analysis results indicated that crovalimab exhibited notable efficacy in both primary endpoints. The mean proportion of patients with hemolysis control was an impressive 78.7%, and an encouraging 51.0% of patients achieved transfusion avoidance during the study period. Crovalimab treatment yielded rapid and sustained control of intravascular hemolysis, with LDH levels consistently maintained below 1.5 times ULN from as early as Week 3 through Week 25. Additionally, patients experienced significant improvements in hemoglobin stabilization, a crucial factor in alleviating anemia, and reported substantial reductions in fatigue. Importantly, the safety profile of crovalimab was consistent with the known safety profile of C5 inhibitors, with no treatment-related discontinuations, reaffirming its suitability for use in PNH management.
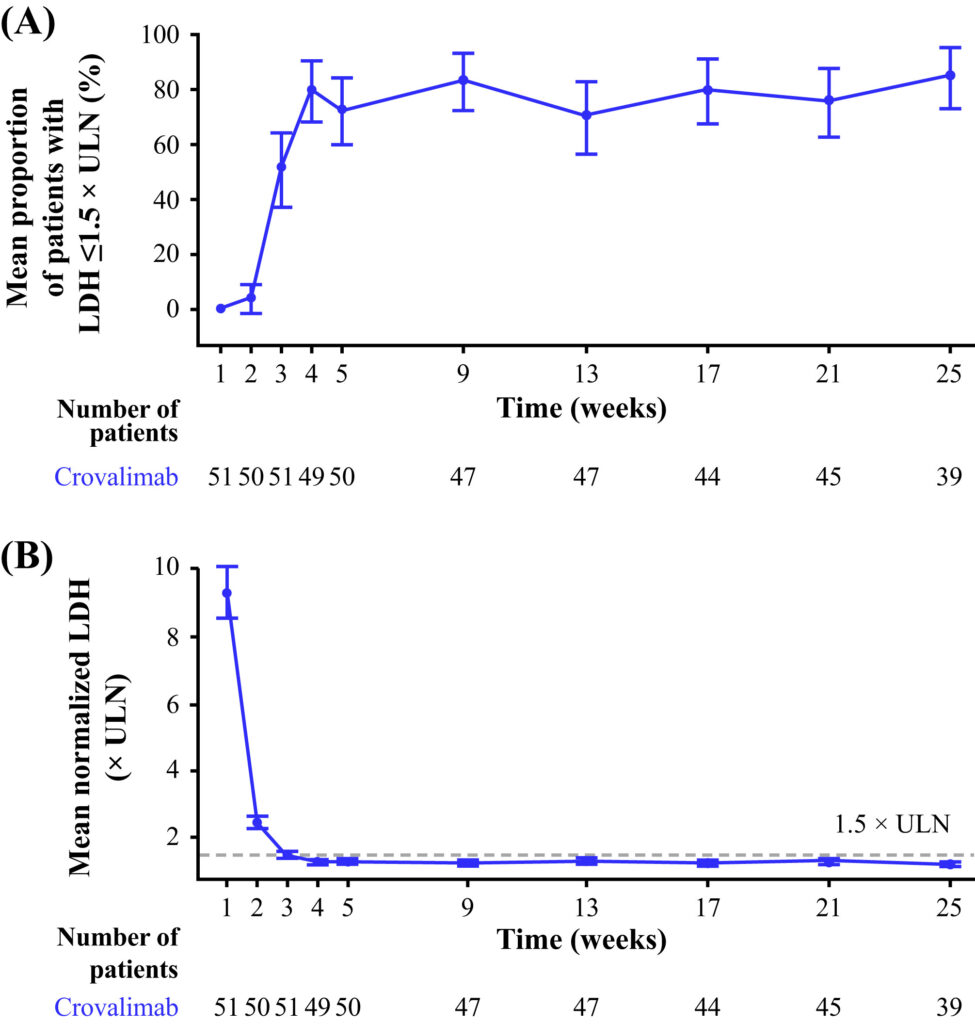
(Am J Hematol,2023 Sep;98(9):1407-1414.)
Crovalimab emerges as a beacon of hope for PNH patients in areas where the standard of care may be limited or unavailable. Its self-administration capability and convenient every-4-week dosing schedule hold the potential to substantially improve the quality of life for these patients. The robust efficacy and safety profile of crovalimab make it an essential addition to the treatment arsenal for PNH, offering a valuable alternative to existing C5 inhibitors. Future studies that compare crovalimab with other complement inhibitors and evaluate its long-term effects are warranted, paving the way for further advancements in the management of PNH.
In conclusion, the findings from this study establish crovalimab as a highly effective and well-tolerated therapeutic option for complement inhibitor-naive patients grappling with the challenges of PNH. Crovalimab’s ability to rapidly and durably control intravascular hemolysis, ultimately leading to transfusion avoidance, marks a significant milestone in PNH treatment. Moreover, the observed improvements in hemoglobin levels and reductions in fatigue underscore the broader impact of crovalimab on patients’ quality of life. Crovalimab not only provides hope but also a promising solution for patients facing the complexities of PNH, especially those in regions with limited access to standard complement inhibitors.


