In February 2023, a study led by Professor Rong Fu from Tianjin Medical University General Hospital was published in the prestigious international academic journal —— Advanced Materials(IF=32.086). The title of the study is "BSA-AIE Nanoparticles with Boosted ROS Generation for Immunogenic Cell Death Immunotherapy of Multiple Myeloma". The study introduce that BSA/TPA-Erdn nanoparticles represent a promising and innovative strategy for immunogenic cell death immunotherapy of multiple myeloma.
Multiple myeloma (MM) is a hematological cancer characterized by the proliferation of malignant plasma cells in the bone marrow. Despite advancements in MM treatment, relapses and the spread of cancerous cells outside the bone marrow remain challenging. One obstacle is the compromised immune microenvironment surrounding MM tumors, where immune dysfunction and immunosuppression contribute to disease progression and relapse. Immune checkpoint inhibitors, such as programmed cell death 1 (PD-1) inhibitors, have shown limited clinical benefits in MM patients. Therefore, there is a need for novel immunotherapies that can improve and modulate immune functions in MM patients.
The objective of this study is to develop a novel strategy for immunogenic cell death (ICD) immunotherapy using BSA-AIE nanoparticles. The study aims to enhance ROS generation and restore the immune microenvironment in MM tumors, ultimately improving patient outcomes.
Methods:
1. Synthesis of TPA-Erdn Photosensitizer:
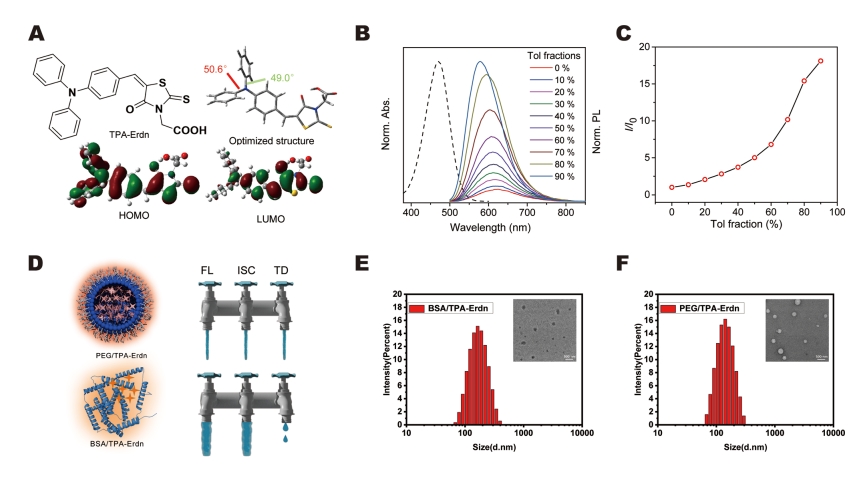
– A novel photosensitizer, TPA-Erdn, with aggregation-induced emission (AIE) properties, is synthesized. TPA-Erdn is designed to improve ROS generation and enhance the intersystem crossing process for efficient T1 formation.
2. Formulation of BSA/TPA-Erdn Nanoparticles:
– TPA-Erdn is loaded into BSA nanoparticles to form BSA/TPA-Erdn nanoparticles. The hydrophobic domain of BSA proteins immobilizes the TPA-Erdn molecules and enhances ROS generation.
3. ROS Generation Capacity:
– The ROS generation capacity of BSA/TPA-Erdn is evaluated and compared to conventional polymer encapsulation strategies, highlighting the superiority of the BSA-based approach.
4. In Vitro Assessment of ICD:
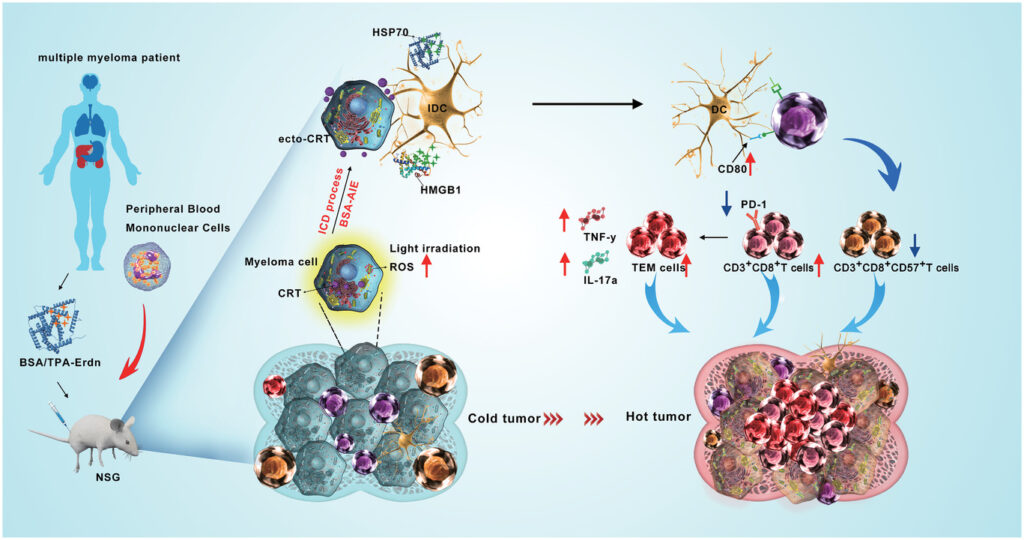
– The ability of BSA/TPA-Erdn to induce immunogenic cell death is assessed in MM cells (RPMI-8226) in vitro. The release of damage-associated molecular patterns (DAMPs), such as ecto-CRT, HMGB1, and HSP70, is measured. The expression of these DAMPs on the cell surface is evaluated, indicating the potential of BSA/TPA-Erdn to stimulate an immune response.
5. Immune Stimulation and T Cell Activation:
– The ability of BSA/TPA-Erdn to stimulate dendritic cell (DC) maturation and T cell activation is investigated using mononuclear cells from MM patients. Co-culture experiments are conducted to evaluate the expression of co-stimulatory molecules, MHC II molecules, and cytokines associated with immune response, demonstrating the immunomodulatory effects of BSA/TPA-Erdn.
6. In Vivo Anticancer Immune Response:
– The anticancer immune response of BSA/TPA-Erdn is evaluated in a mouse model. RPMI-8226 cells are injected into NSG mice, and patient-derived lymphocytes are transferred to establish a human immune system. The efficacy of BSA/TPA-Erdn in inducing immunogenic cell death and stimulating immune responses is assessed. Results indicate the potential of this approach to translate into clinical benefits for MM patients.
(Adv Mater . 2023 Feb;35(7):e2208692.)
BSA/TPA-Erdn nanoparticles exhibit enhanced ROS generation due to the aggregation of AIE photosensitizers within the hydrophobic domain of BSA proteins, highlighting their superior ROS-inducing properties.
BSA/TPA-Erdn effectively induces the release of DAMPs, including ecto-CRT, HMGB1, and HSP70, in MM cells, leading to DC maturation and T cell activation, thereby restoring the immune microenvironment.
BSA/TPA-Erdn shows a higher ability to reverse T cell senescence compared to other treatments, suggesting its potential for rejuvenating the immune response in MM patients.
In vivo studies using an MM mouse model with a human immune system demonstrate that BSA/TPA-Erdn enhances DC maturation, stimulates T cell activation, and reduces the proportion of senescent T cells, underscoring its potential clinical relevance.
The anticancer immune response is improved with increased production of IFN-γ and IL-17a, cytokines associated with anti-tumor activity, indicating the promise of BSA/TPA-Erdn as a therapeutic approach for MM patients.
(Adv Mater . 2023 Feb;35(7):e2208692.)
BSA/TPA-Erdn nanoparticles represent a promising and innovative strategy for immunogenic cell death immunotherapy of multiple myeloma. The enhanced ROS generation and restoration of the immune microenvironment through DC maturation and T cell activation provide new insights and avenues for MM treatment. Moreover, the ability to reverse T cell senescence and stimulate an anti-tumor immune response highlight the remarkable potential of BSA/TPA-Erdn as a therapeutic approach for MM patients. Further studies and clinical trials are warranted to explore the full range of applications and benefits of BSA/TPA-Erdn in MM therapy, with the ultimate goal of improving patient outcomes and quality of life.


